Outer hair cell electromotility and otoacoustic emissions
- PMID: 2187727
- PMCID: PMC2796234
- DOI: 10.1097/00003446-199004000-00003
Outer hair cell electromotility and otoacoustic emissions
Abstract
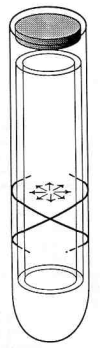
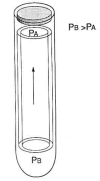
Outer hair cell electromotility is a rapid, force generating, length change in response to electrical stimulation. DC electrical pulses either elongate or shorten the cell and sinusoidal electrical stimulation results in mechanical oscillations at acoustic frequencies. The mechanism underlying outer hair cell electromotility is thought to be the origin of spontaneous otoacoustic emissions. The ability of the cell to change its length requires that it be mechanically flexible. At the same time the structural integrity of the organ of Corti requires that the cell possess considerable compressive rigidity along its major axis. Evolution appears to have arrived at novel solutions to the mechanical requirements imposed on the outer hair cell. Segregation of cytoskeletal elements in specific intracellular domains facilitates the rapid movements. Compressive strength is provided by a unique hydraulic skeleton in which a positive hydrostatic pressure in the cytoplasm stabilizes a flexible elastic cortex with circumferential tensile strength. Cell turgor is required in order that the pressure gradients associated with the electromotile response can be communicated to the ends of the cell. A loss in turgor leads to loss of outer hair cell electromotility. Concentrations of salicylate equivalent to those that abolish spontaneous otoacoustic emissions in patients weaken the outer hair cell's hydraulic skeleton. There is a significant diminution in the electromotile response associated with the loss in cell turgor. Aspirin's effect on outer hair cell electromotility attests to the role of the outer hair cell in generating otoacoustic emissions and demonstrates how their physiology can influence the propagation of otoacoustic emissions.
Figures

References
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. Garland; New York: 1983.
-
- Ashmore JF, Brownell WE. Kilohertz movements induced by electrical stimulation in outer hair cells isolated from the guinea pig cochlea. J Physiol. 1986;377:41P.
-
- Ashmore JF, Meech RW. Ionic basis of membrane potential in outer hair cells of guinea pig cochlea. Nature. 1986;322:368–371. - PubMed
-
- Bannister LH, Dodson HC, Astbury AF, Douek EE. The cortical lattice: a highly order system of subsurface filaments in guinea pig cochlear outer hair cells. Prog Brain Res. 1988;74:213–219. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

