Scaffold diversity of exemplified medicinal chemistry space
- PMID: 21877753
- PMCID: PMC3180201
- DOI: 10.1021/ci2001428
Scaffold diversity of exemplified medicinal chemistry space
Abstract
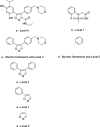
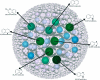
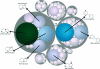
The scaffold diversity of 7 representative commercial and proprietary compound libraries is explored for the first time using both Murcko frameworks and Scaffold Trees. We show that Level 1 of the Scaffold Tree is useful for the characterization of scaffold diversity in compound libraries and offers advantages over the use of Murcko frameworks. This analysis also demonstrates that the majority of compounds in the libraries we analyzed contain only a small number of well represented scaffolds and that a high percentage of singleton scaffolds represent the remaining compounds. We use Tree Maps to clearly visualize the scaffold space of representative compound libraries, for example, to display highly populated scaffolds and clusters of structurally similar scaffolds. This study further highlights the need for diversification of compound libraries used in hit discovery by focusing library enrichment on the synthesis of compounds with novel or underrepresented scaffolds.
Figures


References
-
- Villar H. O.; Hansen M. R. Design of chemical libraries for screening. Expert Opin. Drug Discovery 2009, 4, 1215–1220. - PubMed
-
- Akritopoulou-Zane I.; Hajduk P. J. Kinase-targeted libraries: The design and synthesis of novel, potent, and selective kinase inhibitors. Drug Discovery Today 2009, 14, 291–297. - PubMed
-
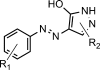
- Markush E. A.Pyrazolone dye and process of making the same, Pharma Chemical Corp, Patent Number: 1506316, United States. 1924. The patent describes the Markush structure as “The yellow coloring matter which may be obtained by coupling to halogen-substitution products of pyrazolone, a diazotized unsulphonated material selected from the group consisting of aniline, homologues of aniline and halogen substitution products of aniline”.
-
- Leach A. R.; Gillet V. J.. An Introduction to Chemoinformatics, Revised ed.; Springer: Dordrecht, 2007.
-
- Brough P. A; Aherne A.; Barril X.; Borgognoni J.; Boxall K.; Cansfield J. E.; Cheung K.-M. J.; Collins I.; Davies N. G. M.; Drysdale M. J.; Dymock B.; Eccles S. A.; Finich H.; Fink A.; Hayes A; Howes R.; Hubbard R. E.; James K.; Jordan A. M.; Lockie A.; Martins V.; Massey A.; Matthews T. P.; McDonald E.; Northfield C. J.; Pearl L. H.; Prodromou C.; Ray S.; Raynaud F. I.; Roughley S. D.; Sharp S. Y.; Surgenor A.; Walmsley D. L.; Webb P.; Wood M.; Workman P.; Wright L. 4, 5-Diarylisoxazole Hsp90 Chaperone Inhibitors: Potential Therapeutic Agents for the Treatment of Cancer. J. Med. Chem. 2008, 51, 196–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

