Wallerian degeneration: the innate-immune response to traumatic nerve injury
- PMID: 21878125
- PMCID: PMC3179447
- DOI: 10.1186/1742-2094-8-109
Wallerian degeneration: the innate-immune response to traumatic nerve injury
Abstract
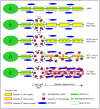
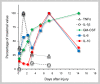
Traumatic injury to peripheral nerves results in the loss of neural functions. Recovery by regeneration depends on the cellular and molecular events of Wallerian degeneration that injury induces distal to the lesion site, the domain through which severed axons regenerate back to their target tissues. Innate-immunity is central to Wallerian degeneration since innate-immune cells, functions and molecules that are produced by immune and non-immune cells are involved. The innate-immune response helps to turn the peripheral nerve tissue into an environment that supports regeneration by removing inhibitory myelin and by upregulating neurotrophic properties. The characteristics of an efficient innate-immune response are rapid onset and conclusion, and the orchestrated interplay between Schwann cells, fibroblasts, macrophages, endothelial cells, and molecules they produce. Wallerian degeneration serves as a prelude for successful repair when these requirements are met. In contrast, functional recovery is poor when injury fails to produce the efficient innate-immune response of Wallerian degeneration.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

