Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function
- PMID: 21878340
- PMCID: PMC3928598
- DOI: 10.1016/j.yjmcc.2011.08.009
Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function
Abstract
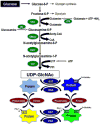
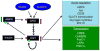
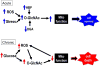
The post-translational modification of serine and threonine residues of nuclear and cytoplasmic proteins by the O-linked attachment of the monosaccharide β-N-acetyl-glucosamine (O-GlcNAc) is emerging as an important mechanism for the regulation of numerous biological processes critical for normal cell function. Active synthesis of O-GlcNAc is essential for cell viability and acute activation of pathways resulting in increased protein O-GlcNAc levels improves the tolerance of cells to a wide range of stress stimuli. Conversely sustained increases in O-GlcNAc levels have been implicated in numerous chronic disease states, especially as a pathogenic contributor to diabetic complications. There has been increasing interest in the role of O-GlcNAc in the heart and vascular system and acute activation of O-GlcNAc levels have been shown to reduce ischemia/reperfusion injury, attenuate vascular injury responses as well mediate some of the detrimental effects of diabetes and hypertension on cardiac and vascular function. Here we provide an overview of our current understanding of pathways regulating protein O-GlcNAcylation, summarize the different methodologies for identifying and characterizing O-GlcNAcylated proteins and subsequently focus on two emerging areas: 1) the role of O-GlcNAc as a potential regulator of cardiac metabolism and 2) the cross talk between O-GlcNAc and reactive oxygen species. This article is part of a Special Section entitled "Post-translational Modification."
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
References
-
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. - PubMed
-
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–17. - PubMed
-
- Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS letters. 2010;584:2526–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

