Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits
- PMID: 21878344
- PMCID: PMC3196356
- DOI: 10.1016/j.neuropharm.2011.08.024
Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits
Abstract
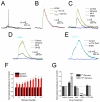
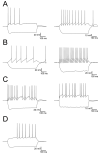
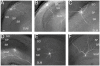
In the hippocampus, activation of nicotinic receptors that include α4 and β2 subunits (α4β2*) facilitates memory formation. α4β2* receptors may also play a role in nicotine withdrawal, and their loss may contribute to cognitive decline in aging and Alzheimer's disease (AD). However, little is known about their cellular function in the hippocampus. Therefore, using optogenetics, whole cell patch clamping and voltage-sensitive dye (VSD) imaging, we measured nicotinic excitatory postsynaptic potentials (EPSPs) in hippocampal CA1. In a subpopulation of inhibitory interneurons, release of ACh resulted in slow depolarizations (rise time constant 33.2 ± 6.5 ms, decay time constant 138.6 ± 27.2 ms) mediated by the activation of α4β2* nicotinic receptors. These interneurons had somata and dendrites located in the stratum oriens (SO) and stratum lacunosum-moleculare (SLM). Furthermore, α4β2* nicotinic EPSPs were largest in the SLM. Thus, our data suggest that nicotinic EPSPs in hippocampal CA1 interneurons are predominantly mediated by α4β2* nicotinic receptors and their activation may preferentially affect extrahippocampal inputs in SLM of hippocampal CA1.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. - PubMed
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J.Pharmacol.Exp.Ther. 1997;283:1396–1411. - PubMed
-
- Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, Cramer SC, Breiter HC, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behav.Neurosci. 2004;118:223–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

