TRAP-1, the mitochondrial Hsp90
- PMID: 21878357
- PMCID: PMC3263322
- DOI: 10.1016/j.bbamcr.2011.08.007
TRAP-1, the mitochondrial Hsp90
Abstract
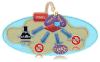
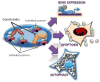
Protein folding quality control does not occur randomly in cells, but requires the action of specialized molecular chaperones compartmentalized in subcellular microenvironments and organelles. Fresh experimental evidence has now linked a mitochondrial-specific Heat Shock Protein-90 (Hsp90) homolog, Tumor Necrosis Factor Receptor-Associated Protein-1 (TRAP-1) to pleiotropic signaling circuitries of organelle integrity and cellular homeostasis. TRAP-1-directed compartmentalized protein folding is broadly exploited in cancer and neurodegenerative diseases, presenting new opportunities for therapeutic intervention in humans. This article is part of a Special Issue entitled: Heat Shock Protein 90 (Hsp90).
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures
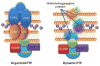
References
-
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. - PubMed
-
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. - PubMed
-
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

