Limb-state information encoded by peripheral and central somatosensory neurons: implications for an afferent interface
- PMID: 21878419
- PMCID: PMC3694199
- DOI: 10.1109/TNSRE.2011.2163145
Limb-state information encoded by peripheral and central somatosensory neurons: implications for an afferent interface
Abstract
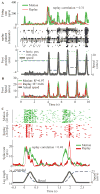
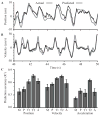
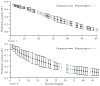
A major issue to be addressed in the development of neural interfaces for prosthetic control is the need for somatosensory feedback. Here, we investigate two possible strategies: electrical stimulation of either dorsal root ganglia (DRG) or primary somatosensory cortex (S1). In each approach, we must determine a model that reflects the representation of limb state in terms of neural discharge. This model can then be used to design stimuli that artificially activate the nervous system to convey information about limb state to the subject. Electrically activating DRG neurons using naturalistic stimulus patterns, modeled on recordings made during passive limb movement, evoked activity in S1 that was similar to that of the original movement. We also found that S1 neural populations could accurately discriminate different patterns of DRG stimulation across a wide range of stimulus pulse-rates. In studying the neural coding in S1, we also decoded the kinematics of active limb movement using multi-electrode recordings in the monkey. Neurons having both proprioceptive and cutaneous receptive fields contributed equally to this decoding. Some neurons were most informative of limb state in the recent past, but many others appeared to signal upcoming movements suggesting that they also were modulated by an efference copy signal. Finally, we show that a monkey was able to detect stimulation through a large percentage of electrodes implanted in area 2. We discuss the design of appropriate stimulus paradigms for conveying time-varying limb state information, and the relative merits and limitations of central and peripheral approaches.
Figures





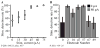
Similar articles
-
Designing stimulation patterns for an afferent BMI: representation of kinetics in somatosensory cortex.Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:7521-4. doi: 10.1109/IEMBS.2011.6091854. Annu Int Conf IEEE Eng Med Biol Soc. 2011. PMID: 22256078
-
Responses of somatosensory area 2 neurons to actively and passively generated limb movements.J Neurophysiol. 2013 Mar;109(6):1505-13. doi: 10.1152/jn.00372.2012. Epub 2012 Dec 28. J Neurophysiol. 2013. PMID: 23274308 Free PMC article.
-
Neuro-fuzzy decoding of sensory information from ensembles of simultaneously recorded dorsal root ganglion neurons for functional electrical stimulation applications.J Neural Eng. 2011 Aug;8(4):046019. doi: 10.1088/1741-2560/8/4/046019. Epub 2011 Jun 23. J Neural Eng. 2011. PMID: 21701057
-
Stimulus-driven changes in sensorimotor behavior and neuronal functional connectivity application to brain-machine interfaces and neurorehabilitation.Prog Brain Res. 2011;192:83-102. doi: 10.1016/B978-0-444-53355-5.00006-3. Prog Brain Res. 2011. PMID: 21763520 Review.
-
Sensory feedback for upper limb prostheses.Prog Brain Res. 2011;192:69-81. doi: 10.1016/B978-0-444-53355-5.00005-1. Prog Brain Res. 2011. PMID: 21763519 Review.
Cited by
-
Selectivity and Longevity of Peripheral-Nerve and Machine Interfaces: A Review.Front Neurorobot. 2017 Oct 31;11:59. doi: 10.3389/fnbot.2017.00059. eCollection 2017. Front Neurorobot. 2017. PMID: 29163122 Free PMC article. Review.
-
Host tissue response to floating microelectrode arrays chronically implanted in the feline spinal nerve.J Neural Eng. 2020 Jul 10;17(4):046012. doi: 10.1088/1741-2552/ab94d7. J Neural Eng. 2020. PMID: 32434161 Free PMC article.
-
Computationally efficient modeling of proprioceptive signals in the upper limb for prostheses: a simulation study.Front Neurosci. 2014 Jun 25;8:181. doi: 10.3389/fnins.2014.00181. eCollection 2014. Front Neurosci. 2014. PMID: 25009463 Free PMC article.
-
Sensory percepts induced by microwire array and DBS microstimulation in human sensory thalamus.Brain Stimul. 2018 Mar-Apr;11(2):416-422. doi: 10.1016/j.brs.2017.10.017. Epub 2017 Oct 27. Brain Stimul. 2018. PMID: 29126946 Free PMC article.
-
Amorphous silicon carbide probe mechanics for insertion in the cerebral cortex of rats, pigs, and macaques.J Neural Eng. 2025 Jun 3;22(3):10.1088/1741-2552/addb79. doi: 10.1088/1741-2552/addb79. J Neural Eng. 2025. PMID: 40398441 Free PMC article.
References
-
- Farrell TR, Weir RF, Heckathorne CW, Childress DS. The effects of static friction and backlash on extended physiological proprioception control of a powered prosthesis. J Rehabil Res Dev. 2005 May-Jun;42:327–41. - PubMed
-
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002 Mar 14;416:141–2. - PubMed
-
- Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999 Jul;2:664–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

