Rearrangement of energetic and substrate utilization networks compensate for chronic myocardial creatine kinase deficiency
- PMID: 21878522
- PMCID: PMC3225674
- DOI: 10.1113/jphysiol.2011.212829
Rearrangement of energetic and substrate utilization networks compensate for chronic myocardial creatine kinase deficiency
Abstract
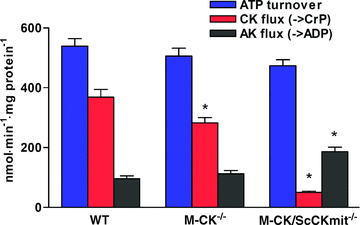
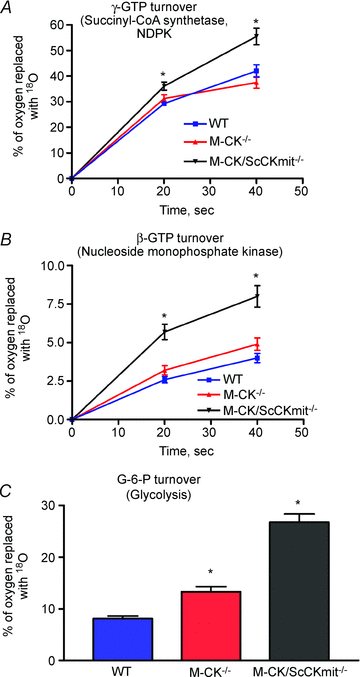
Plasticity of the cellular bioenergetic system is fundamental to every organ function, stress adaptation and disease tolerance. Here, remodelling of phosphotransfer and substrate utilization networks in response to chronic creatine kinase (CK) deficiency, a hallmark of cardiovascular disease, has been revealed in transgenic mouse models lacking either cytosolic M-CK (M-CK(-/-)) or both M-CK and sarcomeric mitochondrial CK (M-CK/ScCKmit(-/-)) isoforms. The dynamic metabolomic signatures of these adaptations have also been defined. Tracking perturbations in metabolic dynamics with (18)O and (13)C isotopes and (31)P NMR and mass spectrometry demonstrate that hearts lacking M-CK have lower phosphocreatine (PCr) turnover but increased glucose-6-phosphate (G-6-P) turnover, glucose utilization and inorganic phosphate compartmentation with normal ATP γ-phosphoryl dynamics. Hearts lacking both M-CK and sarcomeric mitochondrial CK have diminished PCr turnover, total phosphotransfer capacity and intracellular energetic communication but increased dynamics of β-phosphoryls of ADP/ATP, G-6-P and γ-/β-phosphoryls of GTP, indicating redistribution of flux through adenylate kinase (AK), glycolytic and guanine nucleotide phosphotransfer circuits. Higher glycolytic and mitochondrial capacities and increased glucose tolerance contributed to metabolic resilience of M-CK/ScCKmit(-/-) mice. Multivariate analysis revealed unique metabolomic signatures for M-CK(-/-) and M-CK/ScCKmit(-/-) hearts suggesting that rearrangements in phosphotransfer and substrate utilization networks provide compensation for genetic CK deficiency. This new information highlights the significance of integrated CK-, AK-, guanine nucleotide- and glycolytic enzyme-catalysed phosphotransfer networks in supporting the adaptivity and robustness of the cellular energetic system.
Figures


Comment in
-
Seamless networks of myocardial bioenergetics.J Physiol. 2011 Nov 1;589(Pt 21):5013-4. doi: 10.1113/jphysiol.2011.220483. J Physiol. 2011. PMID: 22042542 Free PMC article. No abstract available.
References
-
- Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, et al. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 2002;277:24427–24434. - PubMed
-
- Ashrafian H, Neubauer S. Metabolomic profiling of cardiac substrate utilization: fanning the flames of systems biology? Circulation. 2009;119:1700–1702. - PubMed
-
- Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986;232:1121–1123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

