Mouse model of Timothy syndrome recapitulates triad of autistic traits
- PMID: 21878566
- PMCID: PMC3174658
- DOI: 10.1073/pnas.1112667108
Mouse model of Timothy syndrome recapitulates triad of autistic traits
Abstract
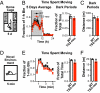
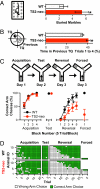
Autism and autism spectrum disorder (ASD) typically arise from a mixture of environmental influences and multiple genetic alterations. In some rare cases, such as Timothy syndrome (TS), a specific mutation in a single gene can be sufficient to generate autism or ASD in most patients, potentially offering insights into the etiology of autism in general. Both variants of TS (the milder TS1 and the more severe TS2) arise from missense mutations in alternatively spliced exons that cause the same G406R replacement in the Ca(V)1.2 L-type calcium channel. We generated a TS2-like mouse but found that heterozygous (and homozygous) animals were not viable. However, heterozygous TS2 mice that were allowed to keep an inverted neomycin cassette (TS2-neo) survived through adulthood. We attribute the survival to lowering of expression of the G406R L-type channel via transcriptional interference, blunting deleterious effects of mutant L-type channel overactivity, and addressed potential effects of altered gene dosage by studying Ca(V)1.2 knockout heterozygotes. Here we present a thorough behavioral phenotyping of the TS2-neo mouse, capitalizing on this unique opportunity to use the TS mutation to model ASD in mice. Along with normal general health, activity, and anxiety level, TS2-neo mice showed markedly restricted, repetitive, and perseverative behavior, altered social behavior, altered ultrasonic vocalization, and enhanced tone-cued and contextual memory following fear conditioning. Our results suggest that when TS mutant channels are expressed at levels low enough to avoid fatality, they are sufficient to cause multiple, distinct behavioral abnormalities, in line with the core aspects of ASD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
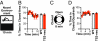
Similar articles
-
Impaired chromaffin cell excitability and exocytosis in autistic Timothy syndrome TS2-neo mouse rescued by L-type calcium channel blockers.J Physiol. 2019 Mar;597(6):1705-1733. doi: 10.1113/JP277487. Epub 2019 Jan 28. J Physiol. 2019. PMID: 30629744 Free PMC article.
-
Disruptions in primary visual cortex physiology and function in a mouse model of Timothy syndrome.Cereb Cortex. 2025 Jun 4;35(6):bhaf162. doi: 10.1093/cercor/bhaf162. Cereb Cortex. 2025. PMID: 40576621 Free PMC article.
-
Enhanced oligodendrocyte maturation and myelination in a mouse model of Timothy syndrome.Glia. 2018 Nov;66(11):2324-2339. doi: 10.1002/glia.23468. Epub 2018 Aug 26. Glia. 2018. PMID: 30151840 Free PMC article.
-
Cav1.2 channelopathies causing autism: new hallmarks on Timothy syndrome.Pflugers Arch. 2020 Jul;472(7):775-789. doi: 10.1007/s00424-020-02430-0. Epub 2020 Jul 3. Pflugers Arch. 2020. PMID: 32621084 Review.
-
L-type Ca2+ channel function during Timothy syndrome.Trends Cardiovasc Med. 2012 Apr;22(3):72-6. doi: 10.1016/j.tcm.2012.06.015. Trends Cardiovasc Med. 2012. PMID: 22999068 Free PMC article. Review.
Cited by
-
Enhanced fear limits behavioral flexibility in Shank2-deficient mice.Mol Autism. 2022 Oct 3;13(1):40. doi: 10.1186/s13229-022-00518-1. Mol Autism. 2022. PMID: 36192805 Free PMC article.
-
Translational outcomes relevant to neurodevelopmental disorders following early life exposure of rats to chlorpyrifos.J Neurodev Disord. 2020 Dec 16;12(1):40. doi: 10.1186/s11689-020-09342-1. J Neurodev Disord. 2020. PMID: 33327943 Free PMC article.
-
Multiple beta cell-independent mechanisms drive hypoglycemia in Timothy syndrome.Nat Commun. 2024 Oct 17;15(1):8980. doi: 10.1038/s41467-024-52885-3. Nat Commun. 2024. PMID: 39420001 Free PMC article.
-
Calcium channelopathies and intellectual disability: a systematic review.Orphanet J Rare Dis. 2021 May 13;16(1):219. doi: 10.1186/s13023-021-01850-0. Orphanet J Rare Dis. 2021. PMID: 33985586 Free PMC article.
-
Autworks: a cross-disease network biology application for Autism and related disorders.BMC Med Genomics. 2012 Nov 28;5:56. doi: 10.1186/1755-8794-5-56. BMC Med Genomics. 2012. PMID: 23190929 Free PMC article.
References
-
- Splawski I, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

