Improved vascular organization enhances functional integration of engineered skeletal muscle grafts
- PMID: 21878567
- PMCID: PMC3169163
- DOI: 10.1073/pnas.1017825108
Improved vascular organization enhances functional integration of engineered skeletal muscle grafts
Erratum in
- Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):1353. Yulia, Shandalov [corrected to Shandalov, Yulia]; Dana, Egozi [corrected to Egozi, Dana]; Daria, Amiad Pavlov [corrected to Pavlov, Daria Amiad]
Abstract
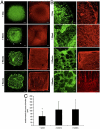
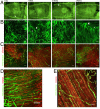
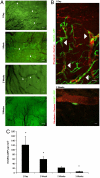
Severe traumatic events such as burns, and cancer therapy, often involve a significant loss of tissue, requiring surgical reconstruction by means of autologous muscle flaps. The scant availability of quality vascularized flaps and donor site morbidity often limit their use. Engineered vascularized grafts provide an alternative for this need. This work describes a first-time analysis, of the degree of in vitro vascularization and tissue organization, required to enhance the pace and efficacy of vascularized muscle graft integration in vivo. While one-day in vitro was sufficient for graft integration, a three-week culturing period, yielding semiorganized vessel structures and muscle fibers, significantly improved grafting efficacy. Implanted vessel networks were gradually replaced by host vessels, coupled with enhanced perfusion and capillary density. Upregulation of key graft angiogenic factors suggest its active role in promoting the angiogenic response. Transition from satellite cells to mature fibers was indicated by increased gene expression, increased capillary to fiber ratio, and similar morphology to normal muscle. We suggest a "relay" approach in which extended in vitro incubation, enabling the formation of a more structured vascular bed, allows for graft-host angiogenic collaboration that promotes anastomosis and vascular integration. The enhanced angiogenic response supports enhanced muscle regeneration, maturation, and integration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Atisha D, Alderman AK. A systematic review of abdominal wall function following abdominal flaps for postmastectomy breast reconstruction. Ann Plast Surg. 2009;63:222–230. - PubMed
-
- Baechler MF, Groth AT, Nesti LJ, Martin BD. Soft tissue management of war wounds to the foot and ankle. Foot Ankle Clin. 2010;15:113–138. http://www.ncbi.nlm.nih.gov/pubmed/19067424. - PMC - PubMed
-
- de Vries Reilingh TS, et al. Autologous tissue repair of large abdominal wall defects. Brit J Surg. 2007;94:791–803. - PubMed
-
- Falco EE, Roth JS, Fisher JP. Skeletal muscle tissue engineering approaches to abdominal wall hernia repair. Birth Defects Res C Embryo Today. 2008;84:315–321. http://www.ncbi.nlm.nih.gov/pubmed?term=20189120%5Buid%5D. - PubMed
-
- Mertsching H, et al. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation. 2009;88:203–210. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

