Forced gating motions by a substituted titratable side chain at the bundle crossing of a potassium channel
- PMID: 21878633
- PMCID: PMC3196073
- DOI: 10.1074/jbc.M111.249110
Forced gating motions by a substituted titratable side chain at the bundle crossing of a potassium channel
Abstract
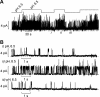
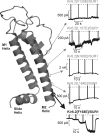
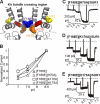
Numerous inwardly rectifying potassium (Kir) channels possess an aromatic residue in the helix bundle crossing region, forming the narrowest pore constriction in crystal structures. However, the role of the Kir channel bundle crossing as a functional gate remains uncertain. We report a unique phenotype of Kir6.2 channels mutated to encode glutamate at this position (F168E). Despite a prediction of four glutamates in close proximity, Kir6.2(F168E) channels are predominantly closed at physiological pH, whereas alkalization causes rapid and reversible channel activation. These findings suggest that F168E glutamates are uncharged at physiological pH but become deprotonated at alkaline pH, forcing channel opening due to mutual repulsion of nearby negatively charged side chains. The potassium channel pore scaffold likely brings these glutamates close together, causing a significant pK(a) shift relative to the free side chain (as seen in the KcsA selectivity filter). Alkalization also shifts the apparent ATP sensitivity of the channel, indicating that forced motion of the bundle crossing is coupled to the ATP-binding site and may resemble conformational changes involved in wild-type Kir6.2 gating. The study demonstrates a novel mechanism for engineering extrinsic control of channel gating by pH and shows that conformational changes in the bundle crossing region are involved in ligand-dependent gating of Kir channels.
Figures



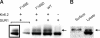


Similar articles
-
Paradoxical activation of an inwardly rectifying potassium channel mutant by spermine: "(b)locking" open the bundle crossing gate.Mol Pharmacol. 2013 Oct;84(4):572-81. doi: 10.1124/mol.113.086603. Epub 2013 Jul 25. Mol Pharmacol. 2013. PMID: 23887925
-
Gating of the ATP-sensitive K+ channel by a pore-lining phenylalanine residue.Biochim Biophys Acta. 2007 Jan;1768(1):39-51. doi: 10.1016/j.bbamem.2006.06.027. Epub 2006 Jul 28. Biochim Biophys Acta. 2007. PMID: 16970907
-
H bonding at the helix-bundle crossing controls gating in Kir potassium channels.Neuron. 2007 Aug 16;55(4):602-14. doi: 10.1016/j.neuron.2007.07.026. Neuron. 2007. PMID: 17698013 Free PMC article.
-
Diverse gating in K+ channels: differential role of the pore-helix glutamate in stabilizing the channel pore.Biochem Biophys Res Commun. 2011 Sep 16;413(1):1-4. doi: 10.1016/j.bbrc.2011.08.062. Epub 2011 Aug 22. Biochem Biophys Res Commun. 2011. PMID: 21872570 Review.
-
Genetic defects in the hotspot of inwardly rectifying K(+) (Kir) channels and their metabolic consequences: a review.Mol Genet Metab. 2012 Jan;105(1):64-72. doi: 10.1016/j.ymgme.2011.10.004. Epub 2011 Oct 19. Mol Genet Metab. 2012. PMID: 22079268 Free PMC article. Review.
Cited by
-
Structure of a KirBac potassium channel with an open bundle crossing indicates a mechanism of channel gating.Nat Struct Mol Biol. 2012 Jan 8;19(2):158-63. doi: 10.1038/nsmb.2208. Nat Struct Mol Biol. 2012. PMID: 22231399 Free PMC article.
-
Decomposition of slide helix contributions to ATP-dependent inhibition of Kir6.2 channels.J Biol Chem. 2013 Aug 9;288(32):23038-49. doi: 10.1074/jbc.M113.485789. Epub 2013 Jun 24. J Biol Chem. 2013. PMID: 23798684 Free PMC article.
-
Inward rectifiers and their regulation by endogenous polyamines.Front Physiol. 2014 Aug 27;5:325. doi: 10.3389/fphys.2014.00325. eCollection 2014. Front Physiol. 2014. PMID: 25221519 Free PMC article. Review.
-
Molecular Dynamics Simulations of KirBac1.1 Mutants Reveal Global Gating Changes of Kir Channels.J Chem Inf Model. 2015 Apr 27;55(4):814-22. doi: 10.1021/acs.jcim.5b00010. Epub 2015 Apr 3. J Chem Inf Model. 2015. PMID: 25794351 Free PMC article.
References
-
- Kubo Y., Adelman J. P., Clapham D. E., Jan L. Y., Karschin A., Kurachi Y., Lazdunski M., Nichols C. G., Seino S., Vandenberg C. A. (2005) Pharmacol. Rev. 57, 509–526 - PubMed
-
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. (1993) Nature 364, 802–806 - PubMed
-
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. (1993) Nature 362, 127–133 - PubMed
-
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. (1993) Nature 362, 31–38 - PubMed
-
- Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. (1995) Science 270, 1166–1170 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

