Inositol pyrophosphates as mammalian cell signals
- PMID: 21878680
- PMCID: PMC3667551
- DOI: 10.1126/scisignal.2001958
Inositol pyrophosphates as mammalian cell signals
Abstract
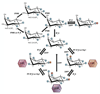
Inositol pyrophosphates are highly energetic inositol polyphosphate molecules present in organisms from slime molds and yeast to mammals. Distinct classes of enzymes generate different forms of inositol pyrophosphates. The biosynthesis of these substances principally involves phosphorylation of inositol hexakisphosphate (IP₆) to generate the pyrophosphate IP₇. Initial insights into functions of these substances derived primarily from yeast, which contain a single isoform of IP₆ kinase (yIP₆K), as well as from the slime mold Dictyostelium. Mammalian functions for inositol pyrophosphates have been investigated by using cell lines to establish roles in various processes, including insulin secretion and apoptosis. More recently, mice with targeted deletion of IP₆K isoforms as well as the related inositol polyphosphate multikinase (IPMK) have substantially enhanced our understanding of inositol polyphosphate physiology. Phenotypic alterations in mice lacking inositol hexakisphosphate kinase 1 (IP₆K1) reveal signaling roles for these molecules in insulin homeostasis, obesity, and immunological functions. Inositol pyrophosphates regulate these processes at least in part by inhibiting activation of the serine-threonine kinase Akt. Similar studies of IP₆K2 establish this enzyme as a cell death inducer acting by stimulating the proapoptotic protein p53. IPMK is responsible for generating the inositol phosphate IP₅ but also has phosphatidylinositol 3-kinase activity--that participates in activation of Akt. Here, we discuss recent advances in understanding the physiological functions of the inositol pyrophosphates based in substantial part on studies in mice with deletion of IP₆K isoforms. These findings highlight the interplay of IPMK and IP₆K in regulating growth factor and nutrient-mediated cell signaling.
Figures

References
-
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. - PubMed
-
- Berridge MJ, Lipp P, Bootman DM. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. - PubMed
-
- Menniti FS, Miller RN, Putney JW, Jr, Shears SB. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J. Biol. Chem. 1993;268:3850–3856. - PubMed
-
- Stephens L, Radenberg T, Thiel U, Vogel G, Khoo KH, Dell A, Jackson TR, Hawkins PT, Mayr GW. The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s) J. Biol. Chem. 1993;268:4009–4015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

