A double-modulation strategy in cancer treatment with a chemotherapeutic agent and siRNA
- PMID: 21878904
- PMCID: PMC3222530
- DOI: 10.1038/mt.2011.174
A double-modulation strategy in cancer treatment with a chemotherapeutic agent and siRNA
Abstract
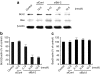
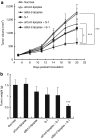
5-Fluorouracil (5-FU) is broadly considered the drug of choice for treating human colorectal cancer (CRC). However, 5-FU resistance, mainly caused by the overexpression of antiapoptotic proteins such as Bcl-2, often leads ultimately to treatment failure. We here investigated the effect of Bcl-2 gene silencing, using small interfering RNA (siRNA) (siBcl-2), on the efficacy of 5-FU in CRC. Transfection of siBcl-2 by a Lipofectamine2000/siRNA lipoplex effectively downregulated Bcl-2 expression in the DLD-1 cell line (a CRC), resulting in significant cell growth inhibition in vitro upon treatment with 5-FU. For in vivo treatments, S-1, an oral formulation of Tegafur (TF), a prodrug of 5-FU, was used to mimic 5-FU infusion. The combined treatment of polyethylene glycol (PEG)-coated siBcl-2-lipoplex and S-1 showed superior tumor growth suppression in a DLD-1 xenograft model, compared to each single treatment. Surprisingly, daily S-1 treatment enhanced the accumulation of PEG-coated siBcl-2-lipoplex in tumor tissue. We propose a novel double modulation strategy in cancer treatment, in which chemotherapy enhances intratumoral siRNA delivery and the delivered siRNA enhances the chemosensitivity of tumors. Combination of siRNA-containing nanocarriers with chemotherapy may compensate for the limited delivery of siRNA to tumor tissue. In addition, such modulation strategy may be considered a promising therapeutic approach to successfully managing 5-FU-resistant tumors.
Figures




References
-
- Subbarayan PR, Sarkar M, Nelson G, Benitez E, Singhal S., and, Ardalan B. Chronic exposure of colorectal cancer cells in culture to fluoropyrimidine analogs induces thymidylate synthase and suppresses p53. A molecular explanation for the mechanism of 5-FU resistance. Anticancer Res. 2010;30:1149–1156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

