Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels
- PMID: 21878911
- PMCID: PMC3265371
- DOI: 10.1038/ncomms1466
Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels
Abstract
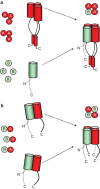
Molecular determinants of ion channel tetramerization are well characterized, but those involved in heteromeric channel assembly are less clearly understood. The heteromeric composition of native channels is often precisely controlled. Cyclic nucleotide-gated (CNG) channels from rod photoreceptors exhibit a 3:1 stoichiometry of CNGA1 and CNGB1 subunits that tunes the channels for their specialized role in phototransduction. Here we show, using electrophysiology, fluorescence, biochemistry, and X-ray crystallography, that the mechanism for this controlled assembly is the formation of a parallel 3-helix coiled-coil domain of the carboxy-terminal leucine zipper region of CNGA1 subunits, constraining the channel to contain three CNGA1 subunits, followed by preferential incorporation of a single CNGB1 subunit. Deletion of the carboxy-terminal leucine zipper domain relaxed the constraint and permitted multiple CNGB1 subunits in the channel. The X-ray crystal structures of the parallel 3-helix coiled-coil domains of CNGA1 and CNGA3 subunits were similar, suggesting that a similar mechanism controls the stoichiometry of cone CNG channels.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
The structure of the native CNGA1/CNGB1 CNG channel from bovine retinal rods.Nat Struct Mol Biol. 2022 Jan;29(1):32-39. doi: 10.1038/s41594-021-00700-8. Epub 2021 Dec 30. Nat Struct Mol Biol. 2022. PMID: 34969975
-
Retinal Cyclic Nucleotide-Gated Channel Regulation by Calmodulin.Int J Mol Sci. 2022 Nov 16;23(22):14143. doi: 10.3390/ijms232214143. Int J Mol Sci. 2022. PMID: 36430626 Free PMC article. Review.
-
Subunit contributions to phosphorylation-dependent modulation of bovine rod cyclic nucleotide-gated channels.J Physiol. 2003 Oct 15;552(Pt 2):345-56. doi: 10.1113/jphysiol.2003.047167. J Physiol. 2003. PMID: 14561819 Free PMC article.
-
Cyclic nucleotide-gated channel subunit glycosylation regulates matrix metalloproteinase-dependent changes in channel gating.Biochemistry. 2013 Nov 19;52(46):8352-62. doi: 10.1021/bi400824x. Epub 2013 Nov 11. Biochemistry. 2013. PMID: 24164424 Free PMC article.
-
Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels.J Biol Chem. 2003 May 23;278(21):18705-8. doi: 10.1074/jbc.R300001200. Epub 2003 Mar 7. J Biol Chem. 2003. PMID: 12626507 Review.
Cited by
-
Deciphering the function of the CNGB1b subunit in olfactory CNG channels.Sci Rep. 2016 Jul 11;6:29378. doi: 10.1038/srep29378. Sci Rep. 2016. PMID: 27405959 Free PMC article.
-
Gating of cyclic nucleotide-gated channels is voltage dependent.Nat Commun. 2012 Jul 24;3:973. doi: 10.1038/ncomms1972. Nat Commun. 2012. PMID: 22828633
-
AAV-Mediated Gene Supplementation Therapy in Achromatopsia Type 2: Preclinical Data on Therapeutic Time Window and Long-Term Effects.Front Neurosci. 2017 May 24;11:292. doi: 10.3389/fnins.2017.00292. eCollection 2017. Front Neurosci. 2017. PMID: 28596720 Free PMC article.
-
Two structural components in CNGA3 support regulation of cone CNG channels by phosphoinositides.J Gen Physiol. 2013 Apr;141(4):413-30. doi: 10.1085/jgp.201210944. J Gen Physiol. 2013. PMID: 23530136 Free PMC article.
-
Cell- and subunit-specific mechanisms of CNG channel ciliary trafficking and localization in C. elegans.J Cell Sci. 2013 Oct 1;126(Pt 19):4381-95. doi: 10.1242/jcs.127274. Epub 2013 Jul 25. J Cell Sci. 2013. PMID: 23886944 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

