Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice
- PMID: 21880018
- PMCID: PMC3242510
- DOI: 10.1042/BJ20110512
Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice
Abstract
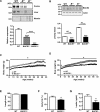
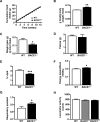
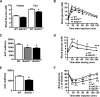
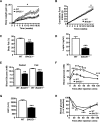
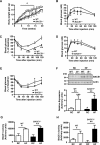
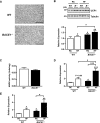
Insulin resistance and impaired glucose homoeostasis are important indicators of Type 2 diabetes and are early risk factors of AD (Alzheimer's disease). An essential feature of AD pathology is the presence of BACE1 (β-site amyloid precursor protein-cleaving enzyme 1), which regulates production of toxic amyloid peptides. However, whether BACE1 also plays a role in glucose homoeostasis is presently unknown. We have used transgenic mice to analyse the effects of loss of BACE1 on body weight, and lipid and glucose homoeostasis. BACE1-/- mice are lean, with decreased adiposity, higher energy expenditure, and improved glucose disposal and peripheral insulin sensitivity than wild-type littermates. BACE1-/- mice are also protected from diet-induced obesity. BACE1-deficient skeletal muscle and liver exhibit improved insulin sensitivity. In a skeletal muscle cell line, BACE1 inhibition increased glucose uptake and enhanced insulin sensitivity. The loss of BACE1 is associated with increased levels of UCP1 (uncoupling protein 1) in BAT (brown adipose tissue) and UCP2 and UCP3 mRNA in skeletal muscle, indicative of increased uncoupled respiration and metabolic inefficiency. Thus BACE1 levels may play a critical role in glucose and lipid homoeostasis in conditions of chronic nutrient excess. Therefore strategies that ameliorate BACE1 activity may be important novel approaches for the treatment of diabetes.
Figures


References
-
- Biessels G. J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. - PubMed
-
- Janson J., Laedtke T., Parisi J. E., O'Brien P., Petersen R. C., Butler P. C. Increased risk of Type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. - PubMed
-
- Craft S., Peskind E., Schwartz M. W., Schellenberg G. D., Raskind M., Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

