Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study
- PMID: 21880132
- PMCID: PMC3179720
- DOI: 10.1186/1748-717X-6-105
Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study
Abstract
Background: Preoperative capecitabine-based chemoradiation is a standard treatment for locally advanced rectal cancer (LARC). Here, we explored the safety and efficacy of the addition of bevacizumab to capecitabine and concurrent radiotherapy for LARC.
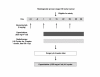
Methods: Patients with MRI-confirmed stage II/III rectal cancer received bevacizumab 5 mg/kg i.v. 2 weeks prior to neoadjuvant chemoradiotherapy followed by bevacizumab 5 mg/kg on Days 1, 15 and 29, capecitabine 825 mg/m2 twice daily on Days 1-38, and concurrent radiotherapy 50.4 Gy (1.8 Gy/day, 5 days/week for 5 weeks + three 1.8 Gy/day), starting on Day 1. Total mesorectal excision was scheduled 6-8 weeks after completion of chemoradiotherapy. Tumour regression grades (TRG) were evaluated on surgical specimens according to Dworak. The primary endpoint was pathological complete response (pCR).
Results: 61 patients were enrolled (median age 60 years [range 31-80], 64% male). Twelve patients (19.7%) had T3N0 tumours, 1 patient T2N1, 19 patients (31.1%) T3N1, 2 patients (3.3%) T2N2, 22 patients (36.1%) T3N2 and 5 patients (8.2%) T4N2. Median tumour distance from the anal verge was 6 cm (range 0-11). Grade 3 adverse events included dermatitis (n = 6, 9.8%), proteinuria (n = 4, 6.5%) and leucocytopenia (n = 3, 4.9%). Radical resection was achieved in 57 patients (95%), and 42 patients (70%) underwent sphincter-preserving surgery. TRG 4 (pCR) was recorded in 8 patients (13.3%) and TRG 3 in 9 patients (15.0%). T-, N- and overall downstaging rates were 45.2%, 73.8%, and 73.8%, respectively.
Conclusions: This study demonstrates the feasibility of preoperative chemoradiotherapy with bevacizumab and capecitabine. The observed adverse events of neoadjuvant treatment are comparable with those previously reported, but the pCR rate was lower.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical