Repression of GW/P body components and the RNAi microprocessor impacts primary ciliogenesis in human astrocytes
- PMID: 21880135
- PMCID: PMC3179929
- DOI: 10.1186/1471-2121-12-37
Repression of GW/P body components and the RNAi microprocessor impacts primary ciliogenesis in human astrocytes
Abstract
Background: In most cells, the centriolar component of the centrosome can function as a basal body supporting the formation of a primary cilium, a non-motile sensory organelle that monitors information from the extracellular matrix and relays stimuli into the cell via associated signaling pathways. Defects in the formation and function of primary cilia underlie multiple human diseases and are hallmarks of malignancy. The RNA silencing pathway is involved in the post-transcriptional silencing of > 50% of mRNA that occurs within GW/P bodies. GW/P bodies are found throughout the cytoplasm and previously published live cell imaging data suggested that in a malignant cell type (U2OS), two GW/P bodies reside at the centrosome during interphase. This led us to investigate if a similar relationship exists in primary cells and if the inhibition of the miRNA pathway impairs primary cilium formation.
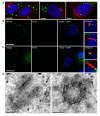
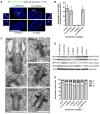
Results: Two GW/P bodies as marked by GW182 and hAgo2 colocalized to the basal body of primary human astrocytes as well as human synoviocytes during interphase and specifically with the distal end of the basal body in the pericentriolar region. Since it is technically challenging to examine the two centrosomal GW/P bodies in isolation, we investigated the potential relationship between the global population of GW/P bodies and primary ciliogenesis. Astrocytes were transfected with siRNA directed to GW182 and hAgo2 and unlike control astrocytes, a primary cilium was no longer associated with the centrosome as detected in indirect immunofluorescence assays. Ultrastructural analysis of siRNA transfected astrocytes revealed that knock down of GW182, hAgo2, Drosha and DGCR8 mRNA did not affect the appearance of the earliest stage of ciliogenesis but did prevent the formation and elongation of the ciliary axoneme.
Conclusions: This study confirms and extends a previously published report that GW/P bodies reside at the centrosome in U2OS cells and documents that GW/P bodies are resident at the centrosome in diverse non-malignant cells. Further, our study demonstrates that repression of key effector proteins in the post-transcriptional miRNA pathway impairs primary cilium formation.
Figures
Similar articles
-
Function of GW182 and GW bodies in siRNA and miRNA pathways.Adv Exp Med Biol. 2013;768:71-96. doi: 10.1007/978-1-4614-5107-5_6. Adv Exp Med Biol. 2013. PMID: 23224966 Review.
-
An SNP in the trinucleotide repeat region of the TNRC6A gene maps to a major TNGW1 autoepitope in patients with autoantibodies to GW182.Adv Exp Med Biol. 2013;768:243-59. doi: 10.1007/978-1-4614-5107-5_14. Adv Exp Med Biol. 2013. PMID: 23224974
-
A role for the P-body component GW182 in microRNA function.Nat Cell Biol. 2005 Dec;7(12):1261-6. doi: 10.1038/ncb1333. Epub 2005 Nov 13. Nat Cell Biol. 2005. PMID: 16284623 Free PMC article.
-
Essential role of Cenexin1, but not Odf2, in ciliogenesis.Cell Cycle. 2013 Feb 15;12(4):655-62. doi: 10.4161/cc.23585. Epub 2013 Jan 23. Cell Cycle. 2013. PMID: 23343771 Free PMC article.
-
GW/P-bodies and autoimmune disease.Adv Exp Med Biol. 2013;768:61-70. doi: 10.1007/978-1-4614-5107-5_5. Adv Exp Med Biol. 2013. PMID: 23224965 Review.
Cited by
-
Centrosome‑, mitotic spindle‑ and cytokinetic bridge‑specific compartmentalization of AGO2 protein in human liver cells undergoing mitosis: Non‑canonical, RNAi‑dependent, control of local homeostasis.Mol Med Rep. 2025 Sep;32(3):244. doi: 10.3892/mmr.2025.13609. Epub 2025 Jul 11. Mol Med Rep. 2025. PMID: 40641140 Free PMC article.
-
Role of primary cilia in non-dividing and post-mitotic cells.Cell Tissue Res. 2017 Jul;369(1):11-25. doi: 10.1007/s00441-017-2599-7. Epub 2017 Mar 30. Cell Tissue Res. 2017. PMID: 28361305 Free PMC article. Review.
-
Spatial and proteomic profiling reveals centrosome-independent features of centriolar satellites.EMBO J. 2019 Jul 15;38(14):e101109. doi: 10.15252/embj.2018101109. Epub 2019 Jun 3. EMBO J. 2019. PMID: 31304627 Free PMC article.
-
Hedgehog pathway, cell cycle, and primary cilium.Cell Death Discov. 2025 Jul 3;11(1):302. doi: 10.1038/s41420-025-02605-7. Cell Death Discov. 2025. PMID: 40610430 Free PMC article. Review.
-
Microtubule and Actin Cytoskeletal Dynamics in Male Meiotic Cells of Drosophila melanogaster.Cells. 2022 Feb 16;11(4):695. doi: 10.3390/cells11040695. Cells. 2022. PMID: 35203341 Free PMC article. Review.
References
-
- Rattner JB, Sciore P, Ou Y, van der Hoorn FA, Lo IKY. Primary cilia in fibroblast-like type B synoviocytes lie within a cilium pit: a site of endocytosis. Histol Histopathol. 2010;25:865–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

