Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group
- PMID: 21880634
- PMCID: PMC3232266
- DOI: 10.3324/haematol.2011.050799
Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group
Abstract
Background: Primary immune thrombocytopenia is a bleeding diathesis with an unknown etiology in predisposed individuals with immune disturbances. Although it is claimed that children and adults differ in clinical and laboratory aspects, few data exist to corroborate this observation. Our objective was to assess comparative data from children and adults with newly diagnosed immune thrombocytopenia.
Design and methods: Clinical and laboratory data of 1,784 children and 340 adults were extracted from the Pediatric and Adult Registry on Chronic Immune Thrombocytopenia. The registry represents a prospective cohort of children and adults with newly diagnosed immune thrombocytopenia. Participating investigators registered their patients immediately after the diagnosis using a web based data transfer. Children aged under 16 years were compared with adults aged 16 years and over with descriptive statistical analyses.
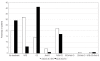
Results: The presenting mean platelet count of children and adults was 18.1 and 25.4 × 10(9)/L. Signs of bleeding were reported in 24% of children and in 23% of adults, and intracranial hemorrhage in 10 of 1,784 children and in 6 of 340 adults. Co-morbidity was observed in 3.9% of children and in 30% of adults. Bone marrow aspiration and laboratory tests (antinuclear antibodies, human immunodeficiency and hepatitis C virus) were performed more frequently in adults. Children and adults were followed with a 'watch and wait' strategy in 20% and in 29%, respectively. Immunoglobulins were used more frequently in children and corticosteroids in adults.
Conclusions: Comparative data of children and adults with newly diagnosed immune thrombocytopenia revealed similarities in presenting platelet counts and in bleeding, whereas differences occurred in co-morbidity, diagnostic procedures and therapy.
Figures

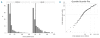

Comment in
-
Immune thrombocytopenia in children and adults: what's the same, what's different?Haematologica. 2011 Dec;96(12):1739-41. doi: 10.3324/haematol.2011.055830. Haematologica. 2011. PMID: 22147771 Free PMC article. No abstract available.
References
-
- Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Op Hematol. 2007;14(5):511–4. - PubMed
-
- Imbach P, Lazarus AH, Kühne T. Intravenous immunoglobulins induce potentially synergistic immunomodulations in autoimmune disorders. Vox Sanguinis. 2010;98(3):385–94. - PubMed
-
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura (ITP) of adults and children. Report from an international working group. Blood. 2009;113(11):2386–93. - PubMed
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2009;85(3):174–80. - PubMed
-
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995–1008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

