Phosphorylation of right open reading frame 2 (Rio2) protein kinase by polo-like kinase 1 regulates mitotic progression
- PMID: 21880710
- PMCID: PMC3196107
- DOI: 10.1074/jbc.M111.250175
Phosphorylation of right open reading frame 2 (Rio2) protein kinase by polo-like kinase 1 regulates mitotic progression
Abstract
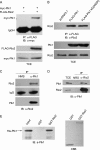
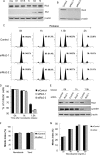
Polo-like kinase 1 (Plk1) plays essential roles during multiple stages of mitosis by phosphorylating a number of substrates. Here, we report that the atypical protein kinase Rio2 is a novel substrate of Plk1 and can be phosphorylated by Plk1 at Ser-335, Ser-380, and Ser-548. Overexpression of Rio2 causes a prolonged mitotic exit whereas knockdown of Rio2 accelerates mitotic progression, suggesting that Rio2 is required for the proper mitotic progression. Overexpression of phospho-mimicking mutant Rio2 S3D but not the nonphosphorylatable mutant Rio2 S3A displays a profile similar to that of wild-type Rio2. These results indicate that the phosphorylation status of Rio2 correlates with its function in mitosis. Furthermore, time-lapse imaging data show that overexpression of Rio2 but not Rio2 S3A results in a slowed metaphase-anaphase transition. Collectively, these findings strongly indicate that the Plk1-mediated phosphorylation of Rio2 regulates metaphase-anaphase transition during mitotic progression.
Figures




Similar articles
-
Phosphorylation of PP1 Regulator Sds22 by PLK1 Ensures Accurate Chromosome Segregation.J Biol Chem. 2016 Sep 30;291(40):21123-21136. doi: 10.1074/jbc.M116.745372. Epub 2016 Aug 24. J Biol Chem. 2016. PMID: 27557660 Free PMC article.
-
PI 3-kinase-dependent phosphorylation of Plk1-Ser99 promotes association with 14-3-3γ and is required for metaphase-anaphase transition.Nat Commun. 2013;4:1882. doi: 10.1038/ncomms2879. Nat Commun. 2013. PMID: 23695676 Free PMC article.
-
MISP is a novel Plk1 substrate required for proper spindle orientation and mitotic progression.J Cell Biol. 2013 Mar 18;200(6):773-87. doi: 10.1083/jcb.201207050. J Cell Biol. 2013. PMID: 23509069 Free PMC article.
-
Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis.Cancer Res. 2006 Jul 15;66(14):6895-8. doi: 10.1158/0008-5472.CAN-06-0358. Cancer Res. 2006. PMID: 16849530 Review.
-
The Mitotic Cancer Target Polo-Like Kinase 1: Oncogene or Tumor Suppressor?Genes (Basel). 2019 Mar 11;10(3):208. doi: 10.3390/genes10030208. Genes (Basel). 2019. PMID: 30862113 Free PMC article. Review.
Cited by
-
SCF(FBXW7α) modulates the intra-S-phase DNA-damage checkpoint by regulating Polo like kinase-1 stability.Oncotarget. 2014 Jun 30;5(12):4370-83. doi: 10.18632/oncotarget.2021. Oncotarget. 2014. PMID: 24970797 Free PMC article.
-
Structural and developmental expression of Ss-riok-2, an RIO protein kinase encoding gene of Strongyloides stercoralis.Sci Rep. 2017 Aug 18;7(1):8693. doi: 10.1038/s41598-017-07991-2. Sci Rep. 2017. PMID: 28821723 Free PMC article.
-
Proteomic Studies of Primary Acute Myeloid Leukemia Cells Derived from Patients Before and during Disease-Stabilizing Treatment Based on All-Trans Retinoic Acid and Valproic Acid.Cancers (Basel). 2021 Apr 29;13(9):2143. doi: 10.3390/cancers13092143. Cancers (Basel). 2021. PMID: 33946813 Free PMC article.
-
A kinome-wide RNAi screen in Drosophila Glia reveals that the RIO kinases mediate cell proliferation and survival through TORC2-Akt signaling in glioblastoma.PLoS Genet. 2013;9(2):e1003253. doi: 10.1371/journal.pgen.1003253. Epub 2013 Feb 14. PLoS Genet. 2013. PMID: 23459592 Free PMC article.
-
RIOK2 phosphorylation by RSK promotes synthesis of the human small ribosomal subunit.PLoS Genet. 2021 Jun 14;17(6):e1009583. doi: 10.1371/journal.pgen.1009583. eCollection 2021 Jun. PLoS Genet. 2021. PMID: 34125833 Free PMC article.
References
-
- Barr F. A., Silljé H. H., Nigg E. A. (2004) Nat. Rev. Mol. Cell Biol. 5, 429–440 - PubMed
-
- Takaki T., Trenz K., Costanzo V., Petronczki M. (2008) Curr. Opin. Cell Biol. 20, 650–660 - PubMed
-
- van Vugt M. A., Medema R. H. (2005) Oncogene 24, 2844–2859 - PubMed
-
- Archambault V., Glover D. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 265–275 - PubMed
-
- Toyoshima-Morimoto F., Taniguchi E., Shinya N., Iwamatsu A., Nishida E. (2001) Nature 410, 215–220 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

