Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype
- PMID: 21880712
- PMCID: PMC3196093
- DOI: 10.1074/jbc.M111.257071
Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype
Abstract
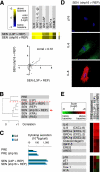
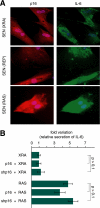
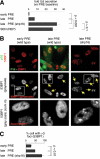
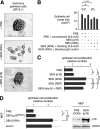
Cellular senescence suppresses cancer by preventing the proliferation of cells that experience potentially oncogenic stimuli. Senescent cells often express p16(INK4a), a cyclin-dependent kinase inhibitor, tumor suppressor, and biomarker of aging, which renders the senescence growth arrest irreversible. Senescent cells also acquire a complex phenotype that includes the secretion of many cytokines, growth factors, and proteases, termed a senescence-associated secretory phenotype (SASP). The SASP is proposed to underlie age-related pathologies, including, ironically, late life cancer. Here, we show that ectopic expression of p16(INK4a) and another cyclin-dependent kinase inhibitor, p21(CIP1/WAF1), induces senescence without a SASP, even though they induced other features of senescence, including a stable growth arrest. Additionally, human fibroblasts induced to senesce by ionizing radiation or oncogenic RAS developed a SASP regardless of whether they expressed p16(INK4a). Cells induced to senesce by ectopic p16(INK4a) expression lacked paracrine activity on epithelial cells, consistent with the absence of a functional SASP. Nonetheless, expression of p16(INK4a) by cells undergoing replicative senescence limited the accumulation of DNA damage and premature cytokine secretion, suggesting an indirect role for p16(INK4a) in suppressing the SASP. These findings suggest that p16(INK4a)-positive cells may not always harbor a SASP in vivo and, furthermore, that the SASP is not a consequence of p16(INK4a) activation or senescence per se, but rather is a damage response that is separable from the growth arrest.
Figures

Similar articles
-
P16 INK4a Deletion Ameliorated Renal Tubulointerstitial Injury in a Stress-induced Premature Senescence Model of Bmi-1 Deficiency.Sci Rep. 2017 Aug 8;7(1):7502. doi: 10.1038/s41598-017-06868-8. Sci Rep. 2017. Retraction in: Sci Rep. 2025 Jan 14;15(1):1921. doi: 10.1038/s41598-025-85803-8. PMID: 28790310 Free PMC article. Retracted.
-
Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts.Mol Cell Biol. 1999 Mar;19(3):2109-17. doi: 10.1128/MCB.19.3.2109. Mol Cell Biol. 1999. PMID: 10022898 Free PMC article.
-
Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity.Aging Cell. 2022 May;21(5):e13602. doi: 10.1111/acel.13602. Epub 2022 Apr 1. Aging Cell. 2022. PMID: 35363946 Free PMC article.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP).Cell Signal. 2012 Apr;24(4):835-45. doi: 10.1016/j.cellsig.2011.12.006. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182507 Review.
Cited by
-
The histone demethylase jumonji coordinates cellular senescence including secretion of neural stem cell-attracting cytokines.Mol Cancer Res. 2015 Apr;13(4):636-50. doi: 10.1158/1541-7786.MCR-13-0268. Epub 2015 Feb 4. Mol Cancer Res. 2015. PMID: 25652587 Free PMC article.
-
Astrocyte senescence as a component of Alzheimer's disease.PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984612 Free PMC article.
-
Aging, cellular senescence, and cancer.Annu Rev Physiol. 2013;75:685-705. doi: 10.1146/annurev-physiol-030212-183653. Epub 2012 Nov 8. Annu Rev Physiol. 2013. PMID: 23140366 Free PMC article. Review.
-
CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, "fueling" tumor growth via paracrine interactions, without an increase in neo-angiogenesis.Cell Cycle. 2012 Oct 1;11(19):3599-610. doi: 10.4161/cc.21884. Epub 2012 Aug 30. Cell Cycle. 2012. PMID: 22935696 Free PMC article.
-
Endothelial senescence-associated secretory phenotype (SASP) is regulated by Makorin-1 ubiquitin E3 ligase.Metabolism. 2019 Nov;100:153962. doi: 10.1016/j.metabol.2019.153962. Epub 2019 Aug 30. Metabolism. 2019. PMID: 31476350 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

