Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement
- PMID: 21880727
- PMCID: PMC3199509
- DOI: 10.1074/jbc.M110.166900
Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement
Abstract
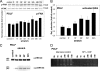
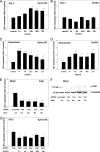
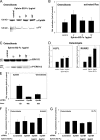
During orthodontic tooth movement, the application of adequate orthodontic forces allows teeth to be moved through the alveolar bone. These forces are transmitted through the periodontal ligaments (PDL) to the supporting alveolar bone and lead to deposition or resorption of bone, depending on whether the tissues are exposed to a tensile or compressive mechanical strain. Fibroblasts within the PDL (PDLF) are considered to be mechanoresponsive. The transduction mechanisms from mechanical loading of the PDLF to the initiation of bone remodeling are not clearly understood. Recently, members of the ephrin/Eph family have been shown to be involved in the regulation of bone homeostasis. For the first time, we demonstrate that PDLF exposed to tensile strain induce the expression of ephrin-B2 via a FAK-, Ras-, ERK1/2-, and SP1-dependent pathway. Osteoblasts of the alveolar bone stimulated with ephrin-B2 increased their osteoblastogenic gene expression and showed functional signs of osteoblastic differentiation. In a physiological setting, ephrin-B2-EphB4 signaling between PDLF and osteoblasts of the alveolar bone might contribute to osteogenesis at tension sites during orthodontic tooth movement.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

