Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers
- PMID: 21880771
- PMCID: PMC3194952
- DOI: 10.1128/JVI.05608-11
Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers
Abstract
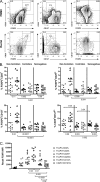
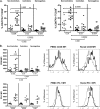
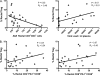
Gut-associated lymphoid tissue (GALT) is a major site of HIV replication and CD4(+) T cell depletion. Furthermore, microbial translocation facilitated by mucosal damage likely contributes to the generalized immune activation observed in HIV infection. Regulatory T cells (Treg) help maintain homeostasis and suppress harmful immune activation during infection; however, in the case of persistent viral infections such as HIV, their role is less clear. Although a number of studies have examined Treg in blood during chronic infection, few have explored Treg in the gastrointestinal mucosa. For this study, paired blood and rectal biopsy samples were obtained from 12 HIV noncontrollers (viral load of >10,000 copies/ml plasma), 10 HIV controllers (viral load of <500 copies/ml plasma for more than 5 years), and 12 HIV seronegative control subjects. Noncontrollers had significantly higher percentages of Treg in rectal mononuclear cells (RMNC), but not in blood, compared to seronegative subjects (P = 0.001) or HIV controllers (P = 0.002). Mucosal Treg positively correlated with viral load (P = 0.01) and expression of immune activation markers by CD4(+) (P = 0.01) and CD8(+) (P = 0.07) T cells. Suppression assays indicated that mucosal and peripheral Treg of noncontrollers and controllers maintained their capacity to suppress non-Treg proliferation to a similar extent as Treg from seronegative subjects. Together, these findings reveal that rather than experiencing depletion, mucosal Treg frequency is enhanced during chronic HIV infection and is positively correlated with viral load and immune activation. Moreover, mucosal Treg maintain their suppressive ability during chronic HIV infection, potentially contributing to diminished HIV-specific T cell responses and viral persistence.
Figures


References
-
- Andersson J., et al. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143–3147 - PubMed
-
- Baecher-Allan C., Brown J. A., Freeman G. J., Hafler D. A. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R24-AI067039/AI/NIAID NIH HHS/United States
- R01-AI057020/AI/NIAID NIH HHS/United States
- P01 AI076174/AI/NIAID NIH HHS/United States
- K23 CA157929/CA/NCI NIH HHS/United States
- C06-RR012088/RR/NCRR NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- R01 AI087145/AI/NIAID NIH HHS/United States
- UL1-RR024131/RR/NCRR NIH HHS/United States
- P01-AI027763/AI/NIAID NIH HHS/United States
- R01-AI076174/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- R01-AI087145/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- R01 AI057020/AI/NIAID NIH HHS/United States
- K24-AI069994/AI/NIAID NIH HHS/United States
- C06 RR012088/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

