Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury
- PMID: 21880831
- PMCID: PMC3233863
- DOI: 10.1152/ajprenal.00332.2011
Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury
Abstract
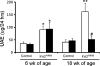
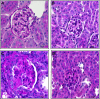
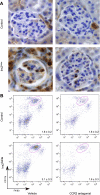
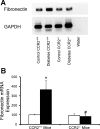
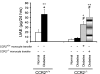
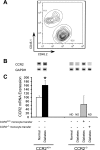
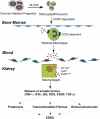
Monocyte/macrophage recruitment correlates strongly with the progression of renal impairment in diabetic nephropathy (DN). C-C chemokine receptor (CCR)2 regulates monocyte/macrophage migration into injured tissues. However, the direct role of CCR2-mediated monocyte/macrophage recruitment in diabetic kidney disease remains unclear. We report that pharmacological blockade or genetic deficiency of CCR2 confers kidney protection in Ins2(Akita) and streptozotocin (STZ)-induced diabetic kidney disease. Blocking CCR2 using the selective CCR2 antagonist RS504393 for 12 wk in Ins2(Akita) mice significantly attenuated albuminuria, the increase in blood urea nitrogen and plasma creatinine, histological changes, and glomerular macrophage recruitment compared with vehicle. Furthermore, mice lacking CCR2 (CCR2(-/-)) mimicked CCR2 blockade by reducing albuminuria and displaying less fibronectin mRNA expression and inflammatory cytokine production compared with CCR2(+/+) mice, despite comparable blood glucose levels. Bone marrow-derived monocytes from CCR2(+/+) or CCR2(-/-) mice adoptively transferred into CCR2(-/-) mice reversed the renal tissue-protective effect in diabetic CCR2(-/-) mice as evaluated by increased urinary albumin excretion and kidney macrophage recruitment, indicating that CCR2 is not required for monocyte migration from the circulation into diabetic kidneys. These findings provide evidence that CCR2 is necessary for monocyte/macrophage-induced diabetic renal injury and suggest that blocking CCR2 could be a novel therapeutic approach in the treatment of DN.
Figures
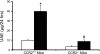

References
-
- Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol 290: F828–F837, 2006 - PubMed
-
- Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

