Essential role for vav Guanine nucleotide exchange factors in brain-derived neurotrophic factor-induced dendritic spine growth and synapse plasticity
- PMID: 21880903
- PMCID: PMC3183742
- DOI: 10.1523/JNEUROSCI.0685-11.2011
Essential role for vav Guanine nucleotide exchange factors in brain-derived neurotrophic factor-induced dendritic spine growth and synapse plasticity
Abstract
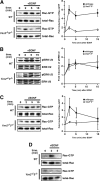
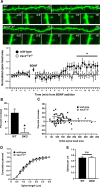
Brain-derived neurotrophic factor (BDNF) and its cognate receptor, TrkB, regulate a wide range of cellular processes, including dendritic spine formation and functional synapse plasticity. However, the signaling mechanisms that link BDNF-activated TrkB to F-actin remodeling enzymes and dendritic spine morphological plasticity remain poorly understood. We report here that BDNF/TrkB signaling in neurons activates the Vav family of Rac/RhoA guanine nucleotide exchange factors through a novel TrkB-dependent mechanism. We find that Vav is required for BDNF-stimulated Rac-GTP production in cortical and hippocampal neurons. Vav is partially enriched at excitatory synapses in the postnatal hippocampus but does not appear to be required for normal dendritic spine density. Rather, we observe significant reductions in both BDNF-induced, rapid, dendritic spine head growth and in CA3-CA1 theta burst-stimulated long-term potentiation in Vav-deficient mouse hippocampal slices, suggesting that Vav-dependent regulation of dendritic spine morphological plasticity facilitates normal functional synapse plasticity.
Figures





References
-
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–10149. - PubMed
-
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. - PubMed
-
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–531. - PubMed
-
- Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–6381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
