Neuronal production of transthyretin in human and murine Alzheimer's disease: is it protective?
- PMID: 21880910
- PMCID: PMC3172869
- DOI: 10.1523/JNEUROSCI.2417-11.2011
Neuronal production of transthyretin in human and murine Alzheimer's disease: is it protective?
Abstract
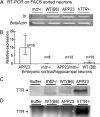
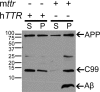
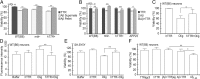
Transthyretin (TTR), a systemic amyloid precursor in the human TTR amyloidoses, interacts with β-amyloid (Aβ) in vitro, inhibits Aβ fibril formation, and suppresses the Alzheimer's disease (AD) phenotype in APP23 mice bearing a human APP gene containing the Swedish autosomal dominant AD mutation. In the present study, we show that TTR is a neuronal product upregulated in AD. Immunohistochemical analysis reveals that, in contrast to brains from non-demented age-matched individuals and control mice, the majority of hippocampal neurons from human AD and all those from the APP23 mouse brains contain TTR. Quantitative PCR for TTR mRNA and Western blot analysis show that primary neurons from APP23 mice transcribe TTR mRNA, and the cells synthesize and secrete TTR protein. TTR mRNA abundance is greatly increased in cultured cortical and hippocampal embryonic neurons and cortical lysates from adult APP23 mice. Antibodies specific for TTR and Aβ pulled down TTR/Aβ complexes from cerebral cortical extracts of APP23 mice and some human AD patients but not from control brains. In complementary tissue culture experiments, recombinant human TTR suppressed the cytotoxicity of soluble Aβ aggregates added to mouse neurons and differentiated human SH-SY5Y neuroblastoma cells. The findings that production of Aβ, its precursor, or its related peptides induces neuronal TTR transcription and synthesis and the presence of Aβ/TTR complexes in vivo suggest that increased TTR production coupled with interaction between TTR and Aβ and/or its related peptides may play a role in natural resistance to human AD.
Figures


Similar articles
-
The systemic amyloid precursor transthyretin (TTR) behaves as a neuronal stress protein regulated by HSF1 in SH-SY5Y human neuroblastoma cells and APP23 Alzheimer's disease model mice.J Neurosci. 2014 May 21;34(21):7253-65. doi: 10.1523/JNEUROSCI.4936-13.2014. J Neurosci. 2014. PMID: 24849358 Free PMC article.
-
Mechanisms of transthyretin inhibition of β-amyloid aggregation in vitro.J Neurosci. 2013 Dec 11;33(50):19423-33. doi: 10.1523/JNEUROSCI.2561-13.2013. J Neurosci. 2013. PMID: 24336709 Free PMC article.
-
Transthyretin accelerates vascular Abeta deposition in a mouse model of Alzheimer's disease.Brain Pathol. 2009 Jan;19(1):48-57. doi: 10.1111/j.1750-3639.2008.00166.x. Epub 2008 Apr 22. Brain Pathol. 2009. PMID: 18429966 Free PMC article.
-
Transthyretin and the brain re-visited: is neuronal synthesis of transthyretin protective in Alzheimer's disease?Mol Neurodegener. 2011 Nov 23;6:79. doi: 10.1186/1750-1326-6-79. Mol Neurodegener. 2011. PMID: 22112803 Free PMC article. Review.
-
The Function of Transthyretin Complexes with Metallothionein in Alzheimer's Disease.Int J Mol Sci. 2020 Nov 26;21(23):9003. doi: 10.3390/ijms21239003. Int J Mol Sci. 2020. PMID: 33256250 Free PMC article. Review.
Cited by
-
Transthyretin provides trophic support via megalin by promoting neurite outgrowth and neuroprotection in cerebral ischemia.Cell Death Differ. 2016 Nov 1;23(11):1749-1764. doi: 10.1038/cdd.2016.64. Epub 2016 Aug 12. Cell Death Differ. 2016. PMID: 27518433 Free PMC article.
-
Stress-responsive regulation of extracellular proteostasis.J Cell Biol. 2022 Apr 4;221(4):e202112104. doi: 10.1083/jcb.202112104. Epub 2022 Feb 22. J Cell Biol. 2022. PMID: 35191945 Free PMC article. Review.
-
TOB is an effector of the hippocampus-mediated acute stress response.Transl Psychiatry. 2022 Jul 29;12(1):302. doi: 10.1038/s41398-022-02078-7. Transl Psychiatry. 2022. PMID: 35906220 Free PMC article.
-
The Relationship of Plasma Transthyretin Level with Global or Regional Amyloid Beta Burden in Subjects with Amnestic Mild Cognitive Impairment: Cross-Sectional Amyloid PET Study.Psychiatry Clin Psychopharmacol. 2022 Mar 1;32(1):4-8. doi: 10.5152/pcp.2022.21206. eCollection 2022 Mar. Psychiatry Clin Psychopharmacol. 2022. PMID: 38764904 Free PMC article.
-
Serum and cerebrospinal fluid levels of transthyretin in Lewy body disorders with and without dementia.PLoS One. 2012;7(10):e48042. doi: 10.1371/journal.pone.0048042. Epub 2012 Oct 25. PLoS One. 2012. PMID: 23133543 Free PMC article.
References
-
- Antequera D, Vargas T, Ugalde C, Spuch C, Molina JA, Ferrer I, Bermejo-Pareja F, Carro E. Cytoplasmic gelsolin increases mitochondrial activity and reduces Abeta burden in a mouse model of Alzheimer's disease. Neurobiol Dis. 2009;36:42–50. - PubMed
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging. 1997;18:S85–S88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
