Saccadic inhibition reveals the timing of automatic and voluntary signals in the human brain
- PMID: 21880912
- PMCID: PMC6703251
- DOI: 10.1523/JNEUROSCI.2234-11.2011
Saccadic inhibition reveals the timing of automatic and voluntary signals in the human brain
Abstract
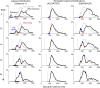
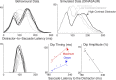
Neurophysiological and phenomenological data on sensorimotor decision making are growing so rapidly that it is now necessary and achievable to capture it in biologically inspired models, for advancing our understanding in both research and clinical settings. However, the main impediment in moving from elegant models with few free parameters to more complex biological models in humans lies in constraining the more numerous parameters with behavioral data (without human single-cell recording). Here we show that a behavioral effect called "saccadic inhibition" (1) is predicted by existing complex (neuronal field) models, (2) constrains crucial temporal parameters of the model, precisely enough to address individual differences, and (3) is not accounted for by current simple decision models, even after significant additions. Visual onsets appearing while an observer plans a saccade knock out a subpopulation of saccadic latencies that would otherwise occur, producing a clear dip in the latency distribution. This overlooked phenomenon is remarkably well time locked across conditions and observers, revealing and characterizing a fast automatic component of visual input to oculomotor competition. The neural field model not only captures this but predicts additional features that are borne out: the dips show spatial specificity, are lawfully modulated in contrast, and occur with S-cone stimuli invisible to the retinotectal route. Overall, we provide a way forward for applying precise neurophysiological models of saccade planning in humans at the individual level.
Figures






References
-
- Antoniades CA, Altham PM, Mason SL, Barker RA, Carpenter R. Saccadometry: a new tool for evaluating presymptomatic Huntington patients. Neuroreport. 2007;18:1133–1136. - PubMed
-
- Bompas A, Sumner P. Sensory sluggishness dissociates saccadic, manual, and perceptual responses: an S-cone study. J Vis. 2008;8:1–13. - PubMed
-
- Bompas A, Sumner P. Oculomotor distraction by signals invisible to the retinotectal and magnocellular pathways. J Neurophysiol. 2009a;102:2387–2395. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
