Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease
- PMID: 21880913
- PMCID: PMC6703266
- DOI: 10.1523/JNEUROSCI.2236-11.2011
Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease
Abstract
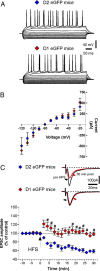
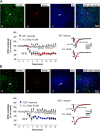
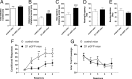
Striatal medium spiny neurons (MSNs) are divided into two subpopulations exerting distinct effects on motor behavior. Transgenic mice carrying bacterial artificial chromosome (BAC) able to confer cell type-specific expression of enhanced green fluorescent protein (eGFP) for dopamine (DA) receptors have been developed to characterize differences between these subpopulations. Analysis of these mice, in contrast with original pioneering studies, showed that striatal long-term depression (LTD) was expressed in indirect but not in the direct pathway MSNs. To address this mismatch, we applied a new approach using combined BAC technology and receptor immunohistochemistry. We demonstrate that, in physiological conditions, DA-dependent LTD is expressed in both pathways showing that the lack of synaptic plasticity found in D(1) eGFP mice is associated to behavioral deficits. Our findings suggest caution in the use of this tool and indicate that the "striatal segregation" hypothesis might not explain all synaptic dysfunctions in Parkinson's disease.
Figures
References
-
- André VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, Levine MS. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010;31:14–28. - PubMed
-
- Bagetta V, Ghiglieri V, Sgobio C, Calabresi P, Picconi B. Synaptic dysfunction in Parkinson's disease. Biochem Soc Trans. 2010;38:493–497. - PubMed
-
- Beckstead RM, Kersey KS. Immunohistochemical demonstration of differential substance P-, met-enkephalin-, and glutamic-acid-decarboxylase-containing cell body and axon distributions in the corpus striatum of the cat. J Comp Neurol. 1985;232:481–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
