Compartmentalization of the GABAB receptor signaling complex is required for presynaptic inhibition at hippocampal synapses
- PMID: 21880914
- PMCID: PMC6703276
- DOI: 10.1523/JNEUROSCI.1527-11.2011
Compartmentalization of the GABAB receptor signaling complex is required for presynaptic inhibition at hippocampal synapses
Erratum in
- J Neurosci. 2012 Feb 15;32(7):2578
Abstract
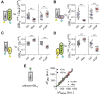
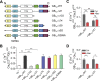
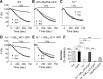
Presynaptic inhibition via G-protein-coupled receptors (GPCRs) and voltage-gated Ca(2+) channels constitutes a widespread regulatory mechanism of synaptic strength. Yet, the mechanism of intermolecular coupling underlying GPCR-mediated signaling at central synapses remains unresolved. Using FRET spectroscopy, we provide evidence for formation of spatially restricted (<100 Å) complexes between GABA(B) receptors composed of GB(1a)/GB(2) subunits, Gα(o)β(1)γ(2) G-protein heterotrimer, and Ca(V)2.2 channels in hippocampal boutons. GABA release was not required for the assembly but for structural reorganization of the precoupled complex. Unexpectedly, GB(1a) deletion disrupted intermolecular associations within the complex. The GB(1a) proximal C-terminal domain was essential for association of the receptor, Ca(V)2.2 and Gβγ, but was dispensable for agonist-induced receptor activation and cAMP inhibition. Functionally, boutons lacking this complex-formation domain displayed impaired presynaptic inhibition of Ca(2+) transients and synaptic vesicle release. Thus, compartmentalization of the GABA(B1a) receptor, Gβγ, and Ca(V)2.2 channel in a signaling complex is required for presynaptic inhibition at hippocampal synapses.
Figures




Similar articles
-
Basal GABA regulates GABA(B)R conformation and release probability at single hippocampal synapses.Neuron. 2010 Jul 29;67(2):253-67. doi: 10.1016/j.neuron.2010.06.022. Neuron. 2010. PMID: 20670833
-
G-protein alpha subunit isoforms couple differentially to receptors that mediate presynaptic inhibition at rat hippocampal synapses.J Neurosci. 2002 Apr 1;22(7):2460-8. doi: 10.1523/JNEUROSCI.22-07-02460.2002. J Neurosci. 2002. PMID: 11923410 Free PMC article.
-
GABAB receptor transduction mechanisms, and cross-talk between protein kinases A and C, in GABAergic terminals synapsing onto neurons of the rat nucleus basalis of Meynert.J Physiol. 2003 Aug 15;551(Pt 1):263-76. doi: 10.1113/jphysiol.2003.046524. Epub 2003 Jun 18. J Physiol. 2003. PMID: 12815184 Free PMC article.
-
GABAB receptor complex as a potential target for tumor therapy.J Histochem Cytochem. 2012 Apr;60(4):269-79. doi: 10.1369/0022155412438105. Epub 2012 Jan 20. J Histochem Cytochem. 2012. PMID: 22266766 Free PMC article. Review.
-
G protein regulation of neuronal calcium channels: back to the future.Mol Pharmacol. 2015 Jun;87(6):890-906. doi: 10.1124/mol.114.096008. Epub 2014 Dec 30. Mol Pharmacol. 2015. PMID: 25549669 Review.
Cited by
-
Synaptotagmin-11 facilitates assembly of a presynaptic signaling complex in post-Golgi cargo vesicles.EMBO Rep. 2024 Jun;25(6):2610-2634. doi: 10.1038/s44319-024-00147-0. Epub 2024 May 2. EMBO Rep. 2024. PMID: 38698221 Free PMC article.
-
Neuronal G protein-gated K+ channels.Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C439-C460. doi: 10.1152/ajpcell.00102.2022. Epub 2022 Jun 15. Am J Physiol Cell Physiol. 2022. PMID: 35704701 Free PMC article. Review.
-
Modulation of neurotransmission by GPCRs is dependent upon the microarchitecture of the primed vesicle complex.J Neurosci. 2014 Jan 1;34(1):260-74. doi: 10.1523/JNEUROSCI.3633-12.2014. J Neurosci. 2014. PMID: 24381287 Free PMC article.
-
Presynaptic GABAB receptors reduce transmission at parabrachial synapses in the lateral central amygdala by inhibiting N-type calcium channels.Sci Rep. 2016 Jan 12;6:19255. doi: 10.1038/srep19255. Sci Rep. 2016. PMID: 26755335 Free PMC article.
-
Association of Rgs7/Gβ5 complexes with Girk channels and GABAB receptors in hippocampal CA1 pyramidal neurons.Hippocampus. 2013 Dec;23(12):1231-45. doi: 10.1002/hipo.22161. Epub 2013 Jul 24. Hippocampus. 2013. PMID: 23804514 Free PMC article.
References
-
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-[beta] as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. - PubMed
-
- Altier C, Zamponi GW. Signaling complexes of voltage-gated calcium channels and G-protein-coupled receptors. J Recept Signal Transduct Res. 2008;28:71–81. - PubMed
-
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. - PubMed
-
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
