Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice
- PMID: 21880920
- PMCID: PMC6703278
- DOI: 10.1523/JNEUROSCI.2397-11.2011
Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice
Abstract
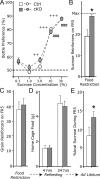
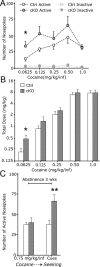
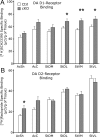
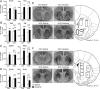
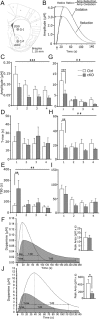
The mesostriatal dopamine (DA) system contributes to several aspects of responses to rewarding substances and is implicated in conditions such as drug addiction and eating disorders. A subset of DA neurons has been shown to express the type 2 Vesicular glutamate transporter (Vglut2) and may therefore corelease glutamate. In the present study, we analyzed mice with a conditional deletion of Vglut2 in DA neurons (Vglut2(f/f;DAT-Cre)) to address the functional significance of the glutamate-DA cophenotype for responses to cocaine and food reinforcement. Biochemical parameters of striatal DA function were also examined by using DA receptor autoradiography, immediate-early gene quantitative in situ hybridization after cocaine challenge, and DA-selective in vivo chronoamperometry. Mice in which Vglut2 expression had been abrogated in DA neurons displayed enhanced operant self-administration of both high-sucrose food and intravenous cocaine. Furthermore, cocaine seeking maintained by drug-paired cues was increased by 76%, showing that reward-dependent plasticity is perturbed in these mice. In addition, several lines of evidence suggest that adaptive changes occurred in both the ventral and dorsal striatum in the absence of VGLUT2: DA receptor binding was increased, and basal mRNA levels of the DA-induced early genes Nur77 and c-fos were elevated as after cocaine induction. Furthermore, in vivo challenge of the DA system by potassium-evoked depolarization revealed less DA release in both striatal areas. This study demonstrates that absence of VGLUT2 in DA neurons leads to perturbations of reward consumption as well as reward-associated memory, features of particular relevance for addictive-like behavior.
Figures
References
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
-
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–4397. - PubMed
-
- Beaudry G, Langlois MC, Weppe I, Rouillard C, Levesque D. Contrasting patterns and cellular specificity of transcriptional regulation of the nuclear receptor nerve growth factor-inducible B by haloperidol and clozapine in the rat forebrain. J Neurochem. 2000;75:1694–1702. - PubMed
-
- Bérubé-Carrière N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
