The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons
- PMID: 21880930
- PMCID: PMC3172708
- DOI: 10.1523/JNEUROSCI.4580-10.2011
The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons
Abstract
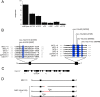
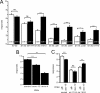
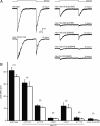
Gentle touch sensation in Caenorhabditis elegans is mediated by the MEC-4/MEC-10 channel complex, which is expressed exclusively in six touch receptor neurons (TRNs). The complex contains two pore-forming subunits, MEC-4 and MEC-10, as well as the accessory subunits MEC-2, MEC-6, and UNC-24. MEC-4 is essential for channel function, but beyond its role as a pore-forming subunit, the functional contribution of MEC-10 to the channel complex and to touch sensation is unclear. We addressed this question using behavioral assays, in vivo electrophysiological recordings from TRNs, and heterologous expression of mutant MEC-10 isoforms. Animals with a deletion in mec-10 showed only a partial loss of touch sensitivity and a modest decrease in the size of the mechanoreceptor current (MRC). In contrast, five previously identified mec-10 alleles acted as recessive gain-of-function alleles that resulted in complete touch insensitivity. Each of these alleles produced a substantial decrease in MRC size and a shift in the reversal potential in vivo. The latter finding indicates that these mec-10 mutations alter the ionic selectivity of the transduction channel in vivo. All mec-10 mutant animals had properly localized channel complexes, indicating that the loss of MRCs was not attributable to a dramatic mislocalization of transduction channels. Finally, electrophysiological examination of heterologously expressed complexes suggests that mutant MEC-10 proteins may affect channel current via MEC-2.
Figures


References
-
- Armstrong CM. Voltage-gated K channels. Sci STKE. 2003;2003:re10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
