Release from the cone ribbon synapse under bright light conditions can be controlled by the opening of only a few Ca(2+) channels
- PMID: 21880934
- PMCID: PMC3234098
- DOI: 10.1152/jn.00634.2011
Release from the cone ribbon synapse under bright light conditions can be controlled by the opening of only a few Ca(2+) channels
Abstract
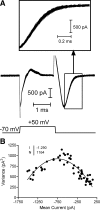
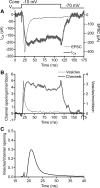
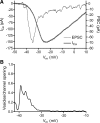
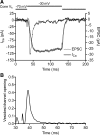
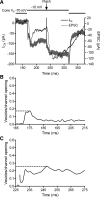
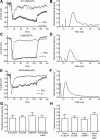
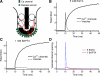
Light hyperpolarizes cone photoreceptors, causing synaptic voltage-gated Ca(2+) channels to open infrequently. To understand neurotransmission under these conditions, we determined the number of L-type Ca(2+) channel openings necessary for vesicle fusion at the cone ribbon synapse. Ca(2+) currents (I(Ca)) were activated in voltage-clamped cones, and excitatory postsynaptic currents (EPSCs) were recorded from horizontal cells in the salamander retina slice preparation. Ca(2+) channel number and single-channel current amplitude were calculated by mean-variance analysis of I(Ca). Two different comparisons-one comparing average numbers of release events to average I(Ca) amplitude and the other involving deconvolution of both EPSCs and simultaneously recorded cone I(Ca)-suggested that fewer than three Ca(2+) channel openings accompanied fusion of each vesicle at the peak of release during the first few milliseconds of stimulation. Opening fewer Ca(2+) channels did not enhance fusion efficiency, suggesting that few unnecessary channel openings occurred during strong depolarization. We simulated release at the cone synapse, using empirically determined synaptic dimensions, vesicle pool size, Ca(2+) dependence of release, Ca(2+) channel number, and Ca(2+) channel properties. The model replicated observations when a barrier was added to slow Ca(2+) diffusion. Consistent with the presence of a diffusion barrier, dialyzing cones with diffusible Ca(2+) buffers did not affect release efficiency. The tight clustering of Ca(2+) channels, along with a high-Ca(2+) affinity release mechanism and diffusion barrier, promotes a linear coupling between Ca(2+) influx and vesicle fusion. This may improve detection of small light decrements when cones are hyperpolarized by bright light.
Figures


References
-
- Ait-Haddou R, Kurachi Y, Nomura T. On calcium-buffer dynamics within the excess buffer regime. J Theor Biol 264: 55–65, 2010 - PubMed
-
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann NY Acad Sci 635: 365–681, 1991 - PubMed
-
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29: 681–690, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

