Mediators of the Jak/STAT signaling pathway in human spermatozoa
- PMID: 21880948
- PMCID: PMC4480430
- DOI: 10.1095/biolreprod.111.092379
Mediators of the Jak/STAT signaling pathway in human spermatozoa
Abstract
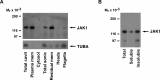
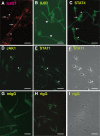
In their journey to acquire the ability to fertilize the egg, numerous intracellular signaling systems are activated in spermatozoa, leading to an increase in protein tyrosine phosphorylation. Although the JAK/STAT signaling pathway is usually associated with the activation of transcription of specific genes, our laboratory previously demonstrated the presence of the IL6 receptor (IL6R) and the Janus kinase 1 (JAK1) in human spermatozoa, a cell that is mostly transcriptionally inactive. In order to determine the importance of the JAK/STAT signaling pathway, our objectives were to identify and characterize the mediators of this system in human sperm. Cell fractionation and surface biotinylation assays clearly demonstrated that IL6R is expressed at the sperm membrane surface. The kinase JAK1 is enriched in membrane fractions and is activated during human sperm capacitation as suggested by its increase in phosphotyrosine content. Many signal transducer and activator of transcription (STAT) proteins are expressed in human sperm, including STAT1, STAT3, STAT4, STAT5, and STAT6. Among them, only STAT1 and STAT5 were detected in the cytosolic fraction. All the detected STAT proteins were enriched in the cytoskeletal structures. STAT4 was present in the perinuclear theca, whereas JAK1, STAT1, and STAT5 were detected in the fibrous sheath. Indirect immunofluorescence studies showed that JAK1 and STAT1 colocalized in the neck region and that STAT4 is present at the equatorial segment and flagella. The presence of STAT proteins in sperm structural components suggests that their role is different from their well-known transcription factor activity in somatic cells, but further investigations are required to determine their role in sperm function.
Figures






Similar articles
-
JAK/STAT/SOCS-signaling pathway and colon and rectal cancer.Mol Carcinog. 2013 Feb;52(2):155-66. doi: 10.1002/mc.21841. Epub 2011 Nov 28. Mol Carcinog. 2013. PMID: 22121102 Free PMC article.
-
Progesterone activates Janus Kinase 1/2 and activators of transcription 1 (JAK1-2/STAT1) pathway in human spermatozoa.Andrologia. 2013 Jun;45(3):178-86. doi: 10.1111/j.1439-0272.2012.01332.x. Epub 2012 Jul 2. Andrologia. 2013. PMID: 22748021
-
Members of the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway are present and active in human sperm.Fertil Steril. 2001 Aug;76(2):258-66. doi: 10.1016/s0015-0282(01)01896-9. Fertil Steril. 2001. PMID: 11476770
-
The role of Janus kinase signaling in the pathology of atopic dermatitis.J Allergy Clin Immunol. 2023 Dec;152(6):1394-1404. doi: 10.1016/j.jaci.2023.07.010. Epub 2023 Aug 1. J Allergy Clin Immunol. 2023. PMID: 37536511 Review.
-
Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches.Drug Resist Updat. 2010 Jun;13(3):67-78. doi: 10.1016/j.drup.2010.04.001. Epub 2010 May 14. Drug Resist Updat. 2010. PMID: 20471303 Review.
Cited by
-
Sub-fertility in crossbred bulls: deciphering testicular level transcriptomic alterations between zebu (Bos indicus) and crossbred (Bos taurus x Bos indicus) bulls.BMC Genomics. 2020 Jul 21;21(1):502. doi: 10.1186/s12864-020-06907-1. BMC Genomics. 2020. PMID: 32693775 Free PMC article.
-
T Cells With Activated STAT4 Drive the High-Risk Rejection State to Renal Allograft Failure After Kidney Transplantation.Front Immunol. 2022 Jul 1;13:895762. doi: 10.3389/fimmu.2022.895762. eCollection 2022. Front Immunol. 2022. PMID: 35844542 Free PMC article.
-
Constructing a seventeen-gene signature model for non-obstructive azoospermia based on integrated transcriptome analyses and WGCNA.Reprod Biol Endocrinol. 2023 Mar 21;21(1):30. doi: 10.1186/s12958-023-01079-5. Reprod Biol Endocrinol. 2023. PMID: 36945018 Free PMC article.
-
IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway.Sci Rep. 2016 Jun 22;6:28012. doi: 10.1038/srep28012. Sci Rep. 2016. PMID: 27329259 Free PMC article.
-
Whole-Genome Profile of Greek Patients with Teratozοοspermia: Identification of Candidate Variants and Genes.Genes (Basel). 2022 Sep 8;13(9):1606. doi: 10.3390/genes13091606. Genes (Basel). 2022. PMID: 36140773 Free PMC article.
References
-
- Yanagimachi R. Mammalian fertilization. : Knobil E, Neil JD. (eds.), The Physiology of Reproduction. New York: Raven Press; 1994: 189 317.
-
- Fraser LR. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev 2010; 77: 197 208. - PubMed
-
- Ellington JE, Samper JC, Jones AE, Oliver SA, Burnett KM, Wright RW. In vitro interactions of cryopreserved stallion spermatozoa and oviduct (uterine tube) epithelial cells or their secretory products. Anim Reprod Sci 1999; 56: 51 65. - PubMed
-
- Laflamme J, Akoum A, Leclerc P. Induction of human sperm capacitation and protein tyrosine phosphorylation by endometrial cells and interleukin-6. Mol Hum Reprod 2005; 11: 141 150. - PubMed
-
- Quintero I, Ghersevich S, Caille A, Munuce MJ, Daniele SM, Morisoli L. Effects of human oviductal in vitro secretion on spermatozoa and search of sperm-oviductal proteins interactions. Int J Androl 2005; 28: 137 143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

