Prevalence and density-related concordance of three diagnostic tests for malaria in a region of Tanzania with hypoendemic malaria
- PMID: 21880972
- PMCID: PMC3209109
- DOI: 10.1128/JCM.01157-11
Prevalence and density-related concordance of three diagnostic tests for malaria in a region of Tanzania with hypoendemic malaria
Abstract
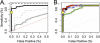
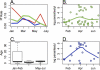
Accurate malaria diagnosis has dual roles in identification of symptomatic persons for effective malaria treatment and also enumeration of asymptomatic persons who contribute to the epidemiologic determinants of transmission. Three currently used diagnostic tests, microscopy, rapid diagnostic tests (RDTs), and real-time PCR, all have different sensitivities and specificities, which are parasite density dependent. Here, we compare their concordance among 451 febrile episodes in a cohort of 2,058 children and adults followed over 6 months in a region in central Tanzania with hypoendemic malaria. Microscopy, a histidine-rich protein-based RDT, and two different real-time PCR gene probes detected Plasmodium falciparum in 20, 54, 41, and 78 episodes of fever, respectively. They had complete concordance in only 9 episodes. Real-time PCR with an 18S probe was more sensitive than with a mitochondrial probe for cytochrome b despite higher copy numbers of mitochondrial DNA. Both PCR yields were increased 4-fold by glycogen/acetate precipitation with low-speed centrifugation. Duplicate PCR increases low-density malaria detection. RDT had the highest number of unique positives, presumably from persistent antigen despite the absence of parasites, although RDT did not detect 3 parasitemias with over 1,000 parasites/μl. In a latent class analysis, real-time PCR had significantly higher sensitivity than did microscopy or RDT. Agreement between real-time PCR, RDT, and microscopy was highest in March and April, when both the P. falciparum parasite rate and parasite densities are highest. Real-time PCR is more sensitive and specific than RDT and microscopy in low-prevalence, low-parasite-density settings.
Figures



References
-
- Beath K.randomLCA: random effects latent class analysis. R package version 0.8-3. 2011. http://CRAN.R-project.org/package=randomLCA.
-
- Bødker R., Msangeni H. A., Kisinza W., Lindsay S. W. 2006. Relationship between the intensity of exposure to malaria parasites and infection in the Usambara Mountains, Tanzania. Am. J. Trop. Med. Hyg. 74:716–723 - PubMed
-
- Brenner H., Gefeller O. 1997. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat. Med. 16:981–991 - PubMed
-
- Maeno Y., et al. 2008. A dried blood sample on filter paper is suitable for detecting Plasmodium falciparum gametocytes by reverse transcription polymerase chain reaction. Acta Trop. 107:121–127 - PubMed
-
- Okell L. C., Ghani A. C., Lyons E., Drakeley C. J. 2009. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J. Infect. Dis. 200:1509–1517 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

