TM4SF10 and ADAP interaction in podocytes: role in Fyn activity and nephrin phosphorylation
- PMID: 21881001
- PMCID: PMC3233801
- DOI: 10.1152/ajpcell.00166.2011
TM4SF10 and ADAP interaction in podocytes: role in Fyn activity and nephrin phosphorylation
Abstract
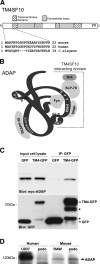
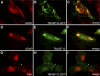
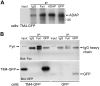
TM4SF10 [transmembrane tetra(4)-span family 10] is a claudin-like cell junction protein that is transiently expressed during podocyte development where its expression is downregulated in differentiating podocytes coincident with the appearance of nephrin at the slit diaphragm. In a yeast two-hybrid screen, we identified adhesion and degranulation-promoting adaptor protein (ADAP), a well-known Fyn substrate and Fyn binding partner, as a TM4SF10 interacting protein in mouse kidney. Using coimmunoprecipitation and immunohistochemistry experiments in cultured human podocytes, we show that TM4SF10 colocalizes with Fyn and ADAP but does not form a stable complex with Fyn. Cytoskeletal changes and phosphorylation events mediated by Fyn activity were reversed by TM4SF10 overexpression, including a decrease in the activating tyrosine phosphorylation of Fyn (Y(421)), suggesting TM4SF10 may have a regulatory role in suppressing Fyn activity. In addition, TM4SF10 was reexpressed following podocyte injury by puromycin aminonucleoside treatment, and its expression enhanced the abundance of high-molecular-weight forms of nephrin indicating it may participate in a mechanism controlling nephrin's appearance at the plasma membrane. Therefore, these studies have identified ADAP as another Fyn adapter protein expressed in podocytes, and that TM4SF10, possibly through ADAP, may regulate Fyn activity. Since TM4SF10 expression is temporally regulated during kidney development, these studies may help define a mechanism by which the slit diaphragm matures as a highly specialized cell junction during podocyte differentiation.
Figures



References
-
- Behrens J, Vakaet L, Friis R, Winterhager E, Van RF, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766, 1993 - PMC - PubMed
-
- Bruggeman LA, Martinka S, Simske JS. Expression of TM4SF10, a Claudin/EMP/PMP22 family cell junction protein, during mouse kidney development and podocyte differentiation. Dev Dyn 236: 596–605, 2007 - PubMed
-
- Christophe-Hobertus C, Szpirer C, Guyon R, Christophe D. Identification of the gene encoding Brain Cell Membrane Protein 1 (BCMP1), a putative four-transmembrane protein distantly related to the peripheral myelin protein 22/epithelial membrane proteins and the claudins. BMC Genomics 2: 3, 2001 - PMC - PubMed
-
- Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci 114: 4307–4318, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

