Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection
- PMID: 21881124
- PMCID: PMC3164429
- DOI: 10.1093/infdis/jir467
Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection
Abstract
Background: Previous studies have shown that the interaction of CD200R, a myeloid inhibitory receptor, with its ligand, CD200, is critical in the control of innate immune activation in the lung.
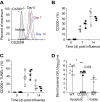
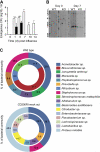
Methods and results: Using a mouse model of bacterial superinfection following influenza, we show that an absence of CD200R (a negative regulator highly expressed by macrophages and dendritic cells), restricts commensal and exogenous bacterial invasiveness and completely prevents the mortality observed in wild-type mice. This benefit is due to a heightened innate immune response to influenza virus in cd200r knockout mice that limits immune pathogenesis and viral load. In wild-type mice, apoptotic cells expressing CD200 that we believe contribute to the suppressed innate immune response to bacteria dominate during the resolution phase of influenza-induced inflammation. We also show for the first time the presence of a variety of previously unidentified bacterial species in the lower airways that are significantly adjusted by influenza virus infection and may contribute to the pathophysiology of disease.
Conclusions: The interaction of CD200 with CD200R therefore contributes to the hyporesponsive innate immune state following influenza virus infection that predisposes to secondary bacterial infection, a phenomenon that has the potential for immune modulation.
Figures
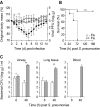
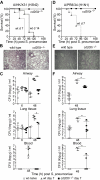
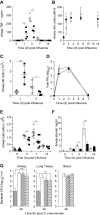
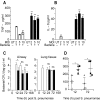
References
-
- Centers for Disease Control and Prevention. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–4. - PubMed
-
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature (London) 1995;377:435–8. - PubMed
-
- McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–9. - PubMed
-
- Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature (London) 2001;409:1055–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

