T cell killing by tolerogenic dendritic cells protects mice from allergy
- PMID: 21881208
- PMCID: PMC3195460
- DOI: 10.1172/JCI45963
T cell killing by tolerogenic dendritic cells protects mice from allergy
Abstract
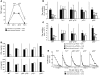
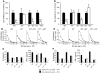
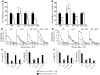
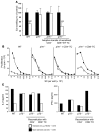
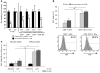
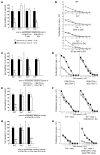
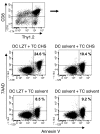
It is well established that allergy development can be prevented by repeated low-dose exposure to contact allergens. Exactly which immune mechanisms are responsible for this so-called low zone tolerance (LZT) is not clear, although CD8⁺ suppressor T cells are known to have a role. Here, we show that TNF released by tolerogenic CD11⁺CD8⁺ DCs located in skin-draining lymph nodes is required and sufficient for development of tolerance to contact allergens in mice. DC-derived TNF protected mice from contact allergy by inducing apoptosis in allergen-specific effector CD8⁺ T cells via TNF receptor 2 but did not contribute to the generation and function of the regulatory T cells associated with LZT. The TNF-mediated killing mechanism was induced in an allergen-specific manner. Activation of tolerogenic DCs by LZT CD8⁺ suppressor T cells and enhanced TNF receptor 2 expression on contact allergen-specific CD8⁺ effector T cells were required for LZT. Our findings may explain how tolerance protects from allergic diseases, which could allow for the development of new strategies for allergy prevention.
Figures
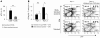

Comment in
-
Allergy: Peaceful death ends pain.Nat Rev Immunol. 2011 Sep 23;11(10):643. doi: 10.1038/nri3076. Nat Rev Immunol. 2011. PMID: 21941293 No abstract available.
Similar articles
-
Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance.J Allergy Clin Immunol. 2012 Sep;130(3):781-797.e11. doi: 10.1016/j.jaci.2012.06.022. J Allergy Clin Immunol. 2012. PMID: 22935591
-
Critical role of IL-10 in the induction of low zone tolerance to contact allergens.J Clin Invest. 2003 Aug;112(3):432-9. doi: 10.1172/JCI18106. Epub 2003 Jul 15. J Clin Invest. 2003. PMID: 12865418 Free PMC article.
-
Low zone tolerance induced by systemic application of allergens inhibits Tc1-mediated skin inflammation.J Allergy Clin Immunol. 2006 May;117(5):1170-7. doi: 10.1016/j.jaci.2006.01.014. Epub 2006 Apr 3. J Allergy Clin Immunol. 2006. PMID: 16675348
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
-
The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review.Front Immunol. 2018 Nov 9;9:2572. doi: 10.3389/fimmu.2018.02572. eCollection 2018. Front Immunol. 2018. PMID: 30473698 Free PMC article. Review.
Cited by
-
Contact dermatitis.Nat Rev Dis Primers. 2021 May 27;7(1):38. doi: 10.1038/s41572-021-00271-4. Nat Rev Dis Primers. 2021. PMID: 34045488 Review.
-
Tolerogenic dendritic cells and TLR4/IRAK4/NF-κB signaling pathway in allergic rhinitis.Front Immunol. 2023 Oct 17;14:1276512. doi: 10.3389/fimmu.2023.1276512. eCollection 2023. Front Immunol. 2023. PMID: 37915574 Free PMC article. Review.
-
Early-Life Nutritional Factors and Mucosal Immunity in the Development of Autoimmune Diabetes.Front Immunol. 2017 Sep 28;8:1219. doi: 10.3389/fimmu.2017.01219. eCollection 2017. Front Immunol. 2017. PMID: 29033938 Free PMC article. Review.
-
The Dendritic Cell Dilemma in the Skin: Between Tolerance and Immunity.Front Immunol. 2022 Jun 28;13:929000. doi: 10.3389/fimmu.2022.929000. eCollection 2022. Front Immunol. 2022. PMID: 35837386 Free PMC article. Review.
-
Allergy: Peaceful death ends pain.Nat Rev Immunol. 2011 Sep 23;11(10):643. doi: 10.1038/nri3076. Nat Rev Immunol. 2011. PMID: 21941293 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

