A sodium channel knockin mutant (NaV1.4-R669H) mouse model of hypokalemic periodic paralysis
- PMID: 21881211
- PMCID: PMC3195470
- DOI: 10.1172/JCI57398
A sodium channel knockin mutant (NaV1.4-R669H) mouse model of hypokalemic periodic paralysis
Abstract
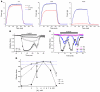
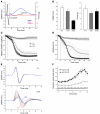
Hypokalemic periodic paralysis (HypoPP) is an ion channelopathy of skeletal muscle characterized by attacks of muscle weakness associated with low serum K+. HypoPP results from a transient failure of muscle fiber excitability. Mutations in the genes encoding a calcium channel (CaV1.1) and a sodium channel (NaV1.4) have been identified in HypoPP families. Mutations of NaV1.4 give rise to a heterogeneous group of muscle disorders, with gain-of-function defects causing myotonia or hyperkalemic periodic paralysis. To address the question of specificity for the allele encoding the NaV1.4-R669H variant as a cause of HypoPP and to produce a model system in which to characterize functional defects of the mutant channel and susceptibility to paralysis, we generated knockin mice carrying the ortholog of the gene encoding the NaV1.4-R669H variant (referred to herein as R669H mice). Homozygous R669H mice had a robust HypoPP phenotype, with transient loss of muscle excitability and weakness in low-K+ challenge, insensitivity to high-K+ challenge, dominant inheritance, and absence of myotonia. Recovery was sensitive to the Na+/K+-ATPase pump inhibitor ouabain. Affected fibers had an anomalous inward current at hyperpolarized potentials, consistent with the proposal that a leaky gating pore in R669H channels triggers attacks, whereas a reduction in the amplitude of action potentials implies additional loss-of-function changes for the mutant NaV1.4 channels.
Figures

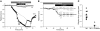


Similar articles
-
Voltage-dependent Ca2+ release is impaired in hypokalemic periodic paralysis caused by CaV1.1-R528H but not by NaV1.4-R669H.Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C478-C485. doi: 10.1152/ajpcell.00209.2022. Epub 2022 Jun 27. Am J Physiol Cell Physiol. 2022. PMID: 35759432 Free PMC article.
-
Disrupted coupling of gating charge displacement to Na+ current activation for DIIS4 mutations in hypokalemic periodic paralysis.J Gen Physiol. 2014 Aug;144(2):137-45. doi: 10.1085/jgp.201411199. Epub 2014 Jul 14. J Gen Physiol. 2014. PMID: 25024265 Free PMC article.
-
Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis.J Gen Physiol. 2008 Oct;132(4):447-64. doi: 10.1085/jgp.200809967. J Gen Physiol. 2008. PMID: 18824591 Free PMC article.
-
Sodium channelopathies of skeletal muscle result from gain or loss of function.Pflugers Arch. 2010 Jul;460(2):239-48. doi: 10.1007/s00424-010-0814-4. Epub 2010 Mar 17. Pflugers Arch. 2010. PMID: 20237798 Free PMC article. Review.
-
Muscle channelopathies: does the predicted channel gating pore offer new treatment insights for hypokalaemic periodic paralysis?J Physiol. 2010 Jun 1;588(Pt 11):1879-86. doi: 10.1113/jphysiol.2009.186627. Epub 2010 Feb 1. J Physiol. 2010. PMID: 20123788 Free PMC article. Review.
Cited by
-
Sodium channelopathies of skeletal muscle and brain.Physiol Rev. 2021 Oct 1;101(4):1633-1689. doi: 10.1152/physrev.00025.2020. Epub 2021 Mar 26. Physiol Rev. 2021. PMID: 33769100 Free PMC article. Review.
-
Skeletal Muscle Channelopathies.Neurotherapeutics. 2018 Oct;15(4):954-965. doi: 10.1007/s13311-018-00678-0. Neurotherapeutics. 2018. PMID: 30341599 Free PMC article. Review.
-
Pathogenic gating pore current conducted by autism-related mutations in the NaV1.2 brain sodium channel.Proc Natl Acad Sci U S A. 2024 Apr 9;121(15):e2317769121. doi: 10.1073/pnas.2317769121. Epub 2024 Apr 2. Proc Natl Acad Sci U S A. 2024. PMID: 38564633 Free PMC article.
-
A novel NaV1.5 voltage sensor mutation associated with severe atrial and ventricular arrhythmias.J Mol Cell Cardiol. 2016 Mar;92:52-62. doi: 10.1016/j.yjmcc.2016.01.014. Epub 2016 Jan 19. J Mol Cell Cardiol. 2016. PMID: 26801742 Free PMC article.
-
Gating-pore currents demonstrate selective and specific modulation of individual sodium channel voltage-sensors by biological toxins.Mol Pharmacol. 2014 Aug;86(2):159-67. doi: 10.1124/mol.114.092338. Epub 2014 Jun 4. Mol Pharmacol. 2014. PMID: 24898004 Free PMC article.
References
-
- Lehmann–Horn F, Rüdel R, Jurkat–Rott K. Nondystrophic myotonias and periodic paralyses. In: Engel AG, Franzini-Armstrong C, eds.Myology . New York, New York, USA: McGraw Hil; 2004:1257–1300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

