Generation and characterization of antibodies specific for caspase-cleaved neo-epitopes: a novel approach
- PMID: 21881607
- PMCID: PMC3186909
- DOI: 10.1038/cddis.2011.91
Generation and characterization of antibodies specific for caspase-cleaved neo-epitopes: a novel approach
Abstract
Apoptosis research has been significantly aided by the generation of antibodies against caspase-cleaved peptide neo-epitopes. However, most of these antibodies recognize the N-terminal fragment and are specific for the protein in question. The aim of this project was to create antibodies, which could identify caspase-cleaved proteins without a priori knowledge of the cleavage sites or even the proteins themselves. We hypothesized that many caspase-cleavage products might have a common antigenic shape, given that they must all fit into the same active site of caspases. Rabbits were immunized with the eight most prevalent exposed C-terminal tetrapeptide sequences following caspase cleavage. After purification of the antibodies we demonstrated (1) their specificity for exposed C-terminal (but not internal) peptides, (2) their ability to detect known caspase-cleaved proteins from apoptotic cell lysates or supernatants from apoptotic cell culture and (3) their ability to detect a caspase-cleaved protein whose tetrapeptide sequence differs from the eight tetrapeptides used to generate the antibodies. These antibodies have the potential to identify novel neo-epitopes produced by caspase cleavage and so can be used to identify pathway-specific caspase cleavage events in a specific cell type. Additionally this methodology may be applied to generate antibodies against products of other proteases, which have a well-defined and non-promiscuous cleavage activity.
Figures
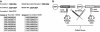
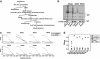


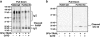
References
-
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. - PubMed
-
- Fulda S. Exploiting apoptosis pathways for the treatment of pediatric cancers. Pediatr Blood Cancer. 2009;53:533–536. - PubMed
-
- Sarker D, Workman P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv Cancer Res. 2007;96:213–268. - PubMed
-
- Amur S, Frueh FW, Lesko LJ, Huang SM. Integration and use of biomarkers in drug development, regulation and clinical practice: a US regulatory perspective. Biomark Med. 2008;2:305–311. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

