Evaluating and reducing the impact of white matter lesions on brain volume measurements
- PMID: 21882300
- PMCID: PMC6870255
- DOI: 10.1002/hbm.21344
Evaluating and reducing the impact of white matter lesions on brain volume measurements
Abstract
MR-based measurements of brain volumes may be affected by the presence of white matter (WM) lesions. Here, we assessed how and to what extent this may happen for WM lesions of various sizes and intensities. After inserting WM lesions of different sizes and intensities into T1-W brain images of healthy subjects, we assessed the effect on two widely used automatic methods for brain volume measurement such as SIENAX (segmentation-based) and SIENA (registration-based). To explore the relevance of partial volume (PV) estimation, we performed the experiments with two different PV models, implemented by the same segmentation algorithm (FAST) of SIENAX and SIENA. Finally, we tested potential solutions to this issue. The presence of WM lesions did not bias measurements for registration-based method such as SIENA. By contrast, the presence of WM lesions affected segmentation-based brain volume measurements such as SIENAx. The misclassification of both gray matter (GM) and WM volumes varied considerably with lesion size and intensity, especially when the lesion intensity was similar to that of the GM/WM interface. The extent to which the presence of WM lesions could affect tissue-class measures was clearly driven by the PV modeling used, with the mixel-type PV model giving a lower error in the presence of WM lesions. The tissue misclassification due to WM lesions was still present when they were masked out. By contrast, refilling the lesions with intensities matching the surrounding normal-appearing WM ensured accurate tissue-class measurements and thus represents a promising approach for accurate tissue classification and brain volume measurements.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
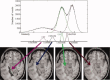

 for GM/WM interface,
for GM/WM interface,  for GM,
for GM,  for CSF/GM and
for CSF/GM and  for CSF). The analysis was performed by using two different partial volume approaches as provided by FAST‐3 (left column) and FAST‐4 (right column) (see Methods for details). Each dot and vertical line in the graphs represents the mean and standard deviations of the five percentage differences obtained by comparing each “original” T1‐weighted image with the related “artificial” T1‐ weighted image of a given lesion load and intensity.
for CSF). The analysis was performed by using two different partial volume approaches as provided by FAST‐3 (left column) and FAST‐4 (right column) (see Methods for details). Each dot and vertical line in the graphs represents the mean and standard deviations of the five percentage differences obtained by comparing each “original” T1‐weighted image with the related “artificial” T1‐ weighted image of a given lesion load and intensity.
 for GM/WM interface,
for GM/WM interface,  for GM,
for GM,  for CSF/WM and
for CSF/WM and  for CSF). The analysis was performed by using two different partial volume approaches as provided by FAST‐3 (left column) and FAST‐4 (right column) (see Methods for details). Each dot and vertical line in the graphs represents the mean and standard deviations of the five percentage differences obtained by comparing each “original” T1‐weighted image with the related “artificial” T1‐ weighted image of a given lesion load and intensity.
for CSF). The analysis was performed by using two different partial volume approaches as provided by FAST‐3 (left column) and FAST‐4 (right column) (see Methods for details). Each dot and vertical line in the graphs represents the mean and standard deviations of the five percentage differences obtained by comparing each “original” T1‐weighted image with the related “artificial” T1‐ weighted image of a given lesion load and intensity.
 ) or “refilled” with the surrounding normal‐appearing WM (
) or “refilled” with the surrounding normal‐appearing WM ( ). Values of NGMV at 0 lesion load are given by those of the “original” T1‐weighed images of the healthy controls. The analysis was performed by using two different partial volume approaches as provided by FAST‐3 (left column) and FAST‐4 (right column) (see Methods for details).
). Values of NGMV at 0 lesion load are given by those of the “original” T1‐weighed images of the healthy controls. The analysis was performed by using two different partial volume approaches as provided by FAST‐3 (left column) and FAST‐4 (right column) (see Methods for details).References
-
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. - PubMed
-
- Battaglini M, Giorgio A, Stromillo ML, Bartolozzi ML, Guidi L, Federico A, De Stefano N ( 2009): Voxel‐wise assessment of progression of regional brain atrophy in relapsing‐remitting multiple sclerosis. J Neurol Sci 282: 55–60 - PubMed
-
- Bendfeldt K, Kuster P, Traud S, Egger H, Winklhofer S, Mueller‐Lenke N, Naegelin Y, Gass A, Kappos L, Matthews PM, Nichols TE, Radue EW, Borgwardt SJ ( 2009): Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis—A longitudinal voxel‐based morphometry study. Neuroimage 45: 60–67. - PubMed
-
- Chard DT, Griffin CM, McLean MA, Kapeller P, Kapoor R, Thompson AJ, Miller DH ( 2002): Brain metabolite changes in cortical grey and normal‐appearing white matter in clinically early relapsing‐remitting multiple sclerosis. Brain 125 ( Part 10): 2342–2352. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

