Effects of a protecting osmolyte on the ion atmosphere surrounding DNA duplexes
- PMID: 21882885
- PMCID: PMC3500086
- DOI: 10.1021/bi200710m
Effects of a protecting osmolyte on the ion atmosphere surrounding DNA duplexes
Abstract
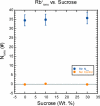
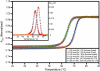
Osmolytes are small, chemically diverse, organic solutes that function as an essential component of cellular stress response. Protecting osmolytes enhance protein stability via preferential exclusion, and nonprotecting osmolytes, such as urea, destabilize protein structures. Although much is known about osmolyte effects on proteins, less is understood about osmolyte effects on nucleic acids and their counterion atmospheres. Nonprotecting osmolytes destabilize nucleic acid structures, but effects of protecting osmolytes depend on numerous factors including the type of nucleic acid and the complexity of the functional fold. To begin quantifying protecting osmolyte effects on nucleic acid interactions, we used small-angle X-ray scattering (SAXS) techniques to monitor DNA duplexes in the presence of sucrose. This protecting osmolyte is a commonly used contrast matching agent in SAXS studies of protein-nucleic acid complexes; thus, it is important to characterize interaction changes induced by sucrose. Measurements of interactions between duplexes showed no dependence on the presence of up to 30% sucrose, except under high Mg(2+) conditions where stacking interactions were disfavored. The number of excess ions associated with DNA duplexes, reported by anomalous small-angle X-ray scattering (ASAXS) experiments, was sucrose independent. Although protecting osmolytes can destabilize secondary structures, our results suggest that ion atmospheres of individual duplexes remain unperturbed by sucrose.
Figures
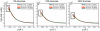
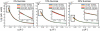


References
-
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. - PubMed
-
- Bolen DW. Protein stabilization by naturally occurring osmolytes. Methods Mol Biol. 2001;168:17–36. - PubMed
-
- Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208:2819–2830. - PubMed
-
- Timasheff SN. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct. 1993;22:67–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

