Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies
- PMID: 21882945
- PMCID: PMC3190966
- DOI: 10.4155/fmc.11.88
Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies
Abstract
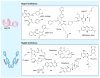
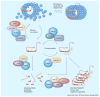
Tau is a microtubule-associated protein that accumulates in at least 15 different neurodegenerative disorders, which are collectively referred to as tauopathies. In these diseases, tau is often hyperphosphorylated and found in aggregates, including paired helical filaments, neurofibrillary tangles and other abnormal oligomers. Tau aggregates are associated with neuron loss and cognitive decline, which suggests that this protein can somehow evade normal quality control allowing it to aberrantly accumulate and become proteotoxic. Consistent with this idea, recent studies have shown that molecular chaperones, such as heat shock protein 70 and heat shock protein 90, counteract tau accumulation and neurodegeneration in disease models. These molecular chaperones are major components of the protein quality control systems and they are specifically involved in the decision to retain or degrade many proteins, including tau and its modified variants. Thus, one potential way to treat tauopathies might be to either accelerate interactions of abnormal tau with these quality control factors or tip the balance of triage towards tau degradation. In this review, we summarize recent findings and suggest models for therapeutic intervention.
Figures

Similar articles
-
Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice.Acta Neuropathol Commun. 2021 Apr 8;9(1):65. doi: 10.1186/s40478-021-01159-w. Acta Neuropathol Commun. 2021. PMID: 33832539 Free PMC article.
-
Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport.Biochemistry. 2011 Nov 29;50(47):10300-10. doi: 10.1021/bi2009147. Epub 2011 Nov 8. Biochemistry. 2011. PMID: 22039833 Free PMC article.
-
Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer's disease.Prog Neurobiol. 2011 Jan;93(1):99-110. doi: 10.1016/j.pneurobio.2010.10.006. Epub 2010 Nov 5. Prog Neurobiol. 2011. PMID: 21056617 Review.
-
Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain.J Neurosci Res. 2007 Nov 1;85(14):3098-108. doi: 10.1002/jnr.21417. J Neurosci Res. 2007. PMID: 17628496
-
Hsp90-interacting Co-chaperones and their Family Proteins in Tau Regulation: Introducing a Novel Role for Cdc37L1.Neuroscience. 2021 Jan 15;453:312-323. doi: 10.1016/j.neuroscience.2020.11.020. Epub 2020 Nov 24. Neuroscience. 2021. PMID: 33246057 Review.
Cited by
-
Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice.Acta Neuropathol Commun. 2021 Apr 8;9(1):65. doi: 10.1186/s40478-021-01159-w. Acta Neuropathol Commun. 2021. PMID: 33832539 Free PMC article.
-
Inhibitors of heat shock protein 70 (Hsp70) with enhanced metabolic stability reduce tau levels.Bioorg Med Chem Lett. 2021 Jun 1;41:128025. doi: 10.1016/j.bmcl.2021.128025. Epub 2021 Apr 9. Bioorg Med Chem Lett. 2021. PMID: 33839251 Free PMC article.
-
Hsp90-Tau complex reveals molecular basis for specificity in chaperone action.Cell. 2014 Feb 27;156(5):963-74. doi: 10.1016/j.cell.2014.01.037. Cell. 2014. PMID: 24581495 Free PMC article.
-
FKBP10 depletion enhances glucocerebrosidase proteostasis in Gaucher disease fibroblasts.Chem Biol. 2013 Mar 21;20(3):403-15. doi: 10.1016/j.chembiol.2012.11.014. Epub 2013 Feb 21. Chem Biol. 2013. PMID: 23434032 Free PMC article.
-
Involvement of T-complex protein 1-ring complex/chaperonin containing T-complex protein 1 (TRiC/CCT) in retrograde axonal transport through tau phosphorylation.Neural Regen Res. 2019 Apr;14(4):588-590. doi: 10.4103/1673-5374.247460. Neural Regen Res. 2019. PMID: 30632495 Free PMC article. No abstract available.
References
-
- Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40(2):139–148. - PubMed
-
- Feany MB, Ksiezak-Reding H, Liu WK, et al. Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol. 1995;90(1):37–43. - PubMed
-
- Goedert M, Spillantini MG, Crowther RA, et al. Tau gene mutation in familial progressive subcortical gliosis. Nat Med. 1999;5(4):454–457. - PubMed
-
- Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia ftdp-17. Nature. 1998;393(6686):702–705. - PubMed
-
- Mattila P, Togo T, Dickson DW. The subthalamic nucleus has neurofibrillary tangles in argyrophilic grain disease and advanced Alzheimer’s disease. Neurosci Lett. 2002;320(1–2):81–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
