Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis
- PMID: 21883043
- PMCID: PMC3324816
- DOI: 10.1089/ars.2011.4243
Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis
Abstract
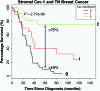
Significance: Here, we review certain recent advances in oxidative stress and tumor metabolism, which are related to understanding the contributions of the microenvironment in promoting tumor growth and metastasis. In the early 1920s, Otto Warburg, a Nobel Laureate, formulated a hypothesis to explain the "fundamental basis" of cancer, based on his observations that tumors displayed a metabolic shift toward glycolysis. In 1963, Christian de Duve, another Nobel Laureate, first coined the phrase auto-phagy, derived from the Greek words "auto" and "phagy," meaning "self" and "eating."
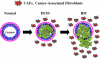
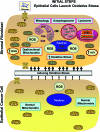
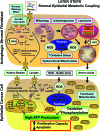
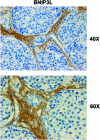
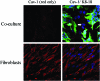
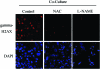
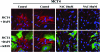
Recent advances: Now, we see that these two ideas (autophagy and aerobic glycolysis) physically converge in the tumor stroma. First, cancer cells secrete hydrogen peroxide. Then, as a consequence, oxidative stress in cancer-associated fibroblasts drives autophagy, mitophagy, and aerobic glycolysis.
Critical issues: This "parasitic" metabolic coupling converts the stroma into a "factory" for the local production of recycled and high-energy nutrients (such as L-lactate)-to fuel oxidative mitochondrial metabolism in cancer cells. We believe that Warburg and de Duve would be pleased with this new two-compartment model for understanding tumor metabolism. It adds a novel stromal twist to two very well-established cancer paradigms: aerobic glycolysis and autophagy.
Future directions: Undoubtedly, these new metabolic models will foster the development of novel biomarkers, and corresponding therapies, to achieve the goal of personalized cancer medicine. Given the central role that oxidative stress plays in this process, new powerful antioxidants should be developed in the fight against cancer.
Figures






References
-
- American Cancer Society. Breast Cancer Facts & Figures 2009–2010. Atlanta: American Cancer Society, Inc.; 2009.
-
- Andre F. Berrada N. Desmedt C. Implication of tumor microenvironment in the resistance to chemotherapy in breast cancer patients. Curr Opin Oncol. 2010;22:547–551. - PubMed
-
- Apel A. Herr I. Schwarz H. Rodemann HP. Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–1494. - PubMed
-
- Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

