Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex
- PMID: 21883374
- PMCID: PMC3708539
- DOI: 10.1111/j.1365-2990.2011.01215.x
Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex
Abstract
Aims: Combined anti-retroviral therapy (cART) has led to a reduction in the incidence of HIV-associated dementia (HAD), a severe motor/cognitive disorder afflicting HIV(+) patients. However, the prevalence of subtler forms of neurocognitive dysfunction, which together with HAD are termed HIV-associated neurocognitive disorders (HAND), continues to escalate in the post-cART era. The microgliosis, astrogliosis, dendritic damage, and synaptic and neuronal loss observed in autopsy cases suggest an underlying neuroinflammatory process, due to the neurotoxic factors released by HIV-infected/activated macrophages/microglia in the brain, might underlie the pathogenesis of HAND in the post-cART era. These factors are known to induce the integrated stress response (ISR) in several neurodegenerative diseases; we have previously shown that BiP, an indicator of general ISR activation, is upregulated in cortical autopsy tissue from HIV-infected patients. The ISR is composed of three pathways, each with its own initiator protein: PERK, IRE1α and ATF6.
Methods: To further elucidate the specific ISR pathways activated in the central nervous system of HAND patients, we examined the protein levels of several ISR proteins, including ATF6, peIF2α and ATF4, in cortical tissue from HIV-infected patients.
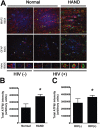
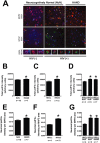
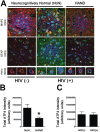
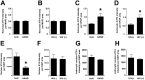
Results: The ISR does not respond in an all-or-none fashion in HAND, but rather demonstrates a nuanced activation pattern. Specifically, our studies implicate the ATF6 pathway of the ISR as a more likely candidate than the PERK pathway for increases in BiP levels in astrocytes.
Conclusion: These findings begin to characterize the nature of the ISR response in HAND and provide potential targets for therapeutic intervention in this disease.
© 2011 The Authors. Neuropathology and Applied Neurobiology © 2011 British Neuropathological Society.
Figures








References
-
- Akay L, Coye MJ. Implementing an employee portal. Taking steps toward a ‘paperless’ future. Healthplan 2001; 42: 66–9 - PubMed
-
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus‐associated dementia: an evolving disease. J Neurovirol 2003; 9: 205–21 - PubMed
-
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long‐standing suppression of viremia. AIDS 2010; 24: 1243–50 - PubMed
-
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV‐1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV‐1‐associated dementia. Curr HIV Res 2003; 1: 463–73 - PubMed
-
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV‐1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 2005; 12 (Suppl. 1): 878–92 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

