Impact of genetic factors (VKORC1, CYP2C9, CYP4F2 and EPHX1) on the anticoagulation response to fluindione
- PMID: 21883387
- PMCID: PMC3370347
- DOI: 10.1111/j.1365-2125.2011.04095.x
Impact of genetic factors (VKORC1, CYP2C9, CYP4F2 and EPHX1) on the anticoagulation response to fluindione
Abstract
Aim: Genetic variants of the enzyme that metabolizes warfarin, cytochrome P-450 2C9 (CYP2C9) and of a key pharmacologic target of vitamin K antagonists, vitamin K epoxide reductase (VKORC1), contribute to differences in patients' responses to coumarin derivatives. The role of these variants in fluindione response is unknown. Our aim was to assess whether genetic factors contribute to the variability in the response to fluindione.
Methods: Four hundred sixty-five patients with a venous thromboembolic event treated by fluindione for at least 3 months with a target international normalized ratio (INR) of 2.0 to 3.0 were studied. VKORC1, CYP2C9, CYP4F2 and EPHX1 genotypes were assessed. INR checks, fluindione doses and bleeding events were collected.
Results: VKORC1 genotype had a significant impact on early anticoagulation (INR value ≥2 after the first two intakes) (P < 0.0001), on the time required to reach a first INR within the therapeutic range (P < 0.0001) and on the time to obtain a first INR value > 4 (P= 0.0002). The average daily dose of fluindione during the first period of stability was significantly associated with the VKORC1 genotype: 19.8 mg (±5.5) for VKORC1 CC, 14.7mg (±6.2) for VKORC1 CT and 8.2mg (±2.5) for VKORC1 TT (P < 0.0001). CYP2C9, CYP4F2 and EPHX1 genotypes did not significantly influence the response to fluindione.
Conclusions: VKORC1 genotype strongly affected anticoagulation induced by fluindione whereas CYP2C9, CYP4F2 and EPHX1 genotypes seemed less determining.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
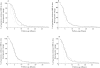
 ); VKORC1 CT (
); VKORC1 CT ( ); VKORC1 TT (
); VKORC1 TT ( ); (B) 2C9 *1/*1 (
); (B) 2C9 *1/*1 ( ); 2C9 *1/*2 or *1/*3 (
); 2C9 *1/*2 or *1/*3 ( ); 2C9 *2/*2 or *2/*3 or *3/*3 (
); 2C9 *2/*2 or *2/*3 or *3/*3 ( ); (C) CYP4F2 CC (
); (C) CYP4F2 CC ( ); CYP4F2 CT (
); CYP4F2 CT ( ); CYP4F2 TT (
); CYP4F2 TT ( ); (D) EPHX1 GG (
); (D) EPHX1 GG ( ); EPHX1 GA (
); EPHX1 GA ( ); EPHX1 AA (
); EPHX1 AA ( )
)
 ); VKORC1 CT (
); VKORC1 CT ( ); VKORC1 TT (
); VKORC1 TT ( ); (B) 2C9 *1/*1 (
); (B) 2C9 *1/*1 ( ); 2C9 *1/*2 or *1/*3 (
); 2C9 *1/*2 or *1/*3 ( ); 2C9 *2/*2 or *2/*3 or *3/*3 (
); 2C9 *2/*2 or *2/*3 or *3/*3 ( ); (C) CYP4F2 CC (
); (C) CYP4F2 CC ( ); CYP4F2 CT (
); CYP4F2 CT ( ); CYP4F2 TT (
); CYP4F2 TT ( ); (D) EPHX1 GG (
); (D) EPHX1 GG ( ); EPHX1 GA (
); EPHX1 GA ( ); EPHX1 AA (
); EPHX1 AA ( )
)References
-
- D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, Grandone E, Margaglione M. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–9. - PubMed
-
- Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, Kesteven P, Daly AK, Kamali F. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–33. - PubMed
-
- Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93. - PubMed
-
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7:97–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

