Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability
- PMID: 21883880
- PMCID: PMC3206146
- DOI: 10.1111/j.1538-7836.2011.04488.x
Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability
Abstract
Background: Microparticles (MPs) are sub-micron vesicles shed by activated or apoptotic cells, including platelets and monocytes. Increased circulating MPs are associated with thrombosis; however, their role in thrombogenesis is poorly understood.
Objective: To determine how MPs promote thrombin generation and modulate fibrin density and stability.
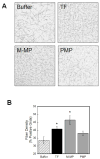
Methods: Platelets and monocytes were isolated from healthy donors. Platelets were stimulated with calcium ionophore, thrombin receptor agonist peptide (TRAP) or TRAP/convulxin. Monocytes and human monocytic THP-1 cells were stimulated with lipopolysaccharide (LPS). MPs were isolated, washed by high-speed centrifugation and assessed using the following: transmission electron microscopy (TEM), Nanoparticle Tracking Analysis (NTA), flow cytometry, tissue factor (TF) activity, prothrombinase activity, thrombin generation, and clot formation, density and stability.
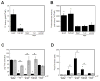
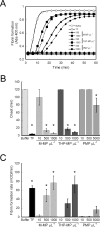
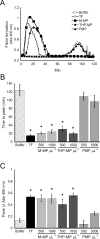
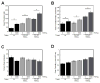
Results: MPs from monocytes (M-MPs) and platelets (PMPs) had similar shapes and diameters (100-300 nm). M-MPs had TF activity (16.7 ± 2.4 pm TF per 10(6) MP), supported prothrombinase activity and triggered shorter thrombin generation lag times than buffer controls (5.4 ± 0.5 vs. 84.2 ± 4.8 min, respectively). Compared with controls, M-MPs supported faster fibrin formation (0.24 ± 0.24 vs. 76.7 ± 15.1 mOD min(-1) , respectively), 38% higher fibrin network density and higher clot stability (3.8-fold higher turbidity in the presence of tissue plasminogen activator). In contrast, PMPs did not have TF activity and supported 2.8-fold lower prothrombinase activity than M-MPs. PMPs supported contact-dependent thrombin generation, but did not independently increase fibrin network density or stability. Interestingly, PMPs increased rates of thrombin generation and fibrin formation (1.7- and 1.3-fold, respectively) when mixed with THP-1-derived MPs.
Conclusion: MPs from platelets and monocytes differentially modulate clot formation, structure and stability, suggesting unique contributions to thrombosis.
© 2011 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
No relevant conflicts of interest to disclose.
Figures

References
-
- Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–46. - PubMed
-
- Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, Horstman LL, Soriano AO, Zambrano JP, Ahn YS. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–71. - PubMed
-
- Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–7. - PubMed
-
- Tripodi A, Branchi A, Chantarangkul V, Clerici M, Merati G, Artoni A, Mannucci PM. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis. 2010 - PubMed
-
- Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

