Altered distribution of interstitial cells and innervation in the rat urinary bladder following spinal cord injury
- PMID: 21883887
- PMCID: PMC3823221
- DOI: 10.1111/j.1582-4934.2011.01410.x
Altered distribution of interstitial cells and innervation in the rat urinary bladder following spinal cord injury
Abstract
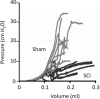
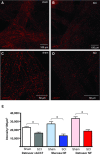
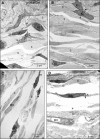
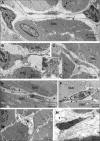
Changes in the distribution of interstitial cells (IC) are reportedly associated with dysfunctional bladder. This study investigated whether spinal cord injury (SCI) resulted in changes to IC subpopulations (vimentin-positive with the ultrastructural profile of IC), smooth muscle and nerves within the bladder wall and correlated cellular remodelling with functional properties. Bladders from SCI (T8/9 transection) and sham-operated rats 5 weeks post-injury were used for ex vivo pressure-volume experiments or processed for morphological analysis with transmission electron microscopy (TEM) and light/confocal microscopy. Pressure-volume relationships revealed low-pressure, hypercompliance in SCI bladders indicative of decompensation. Extensive networks of vimentin-positive IC were typical in sham lamina propria and detrusor but were markedly reduced post-SCI; semi-quantitative analysis showed significant reduction. Nerves labelled with anti-neurofilament and anti-vAChT were notably decreased post-SCI. TEM revealed lamina propria IC and detrusor IC which formed close synaptic-like contacts with vesicle-containing nerve varicosities in shams. Lamina propria and detrusor IC were ultrastructurally damaged post-SCI with retracted/lost cell processes and were adjacent to areas of cellular debris and neuronal degradation. Smooth muscle hypertrophy was common to SCI tissues. In conclusion, IC populations in bladder wall were decreased 5 weeks post-SCI, accompanied with reduced innervation, smooth muscle hypertrophy and increased compliance. These novel findings indicate that bladder wall remodelling post-SCI affects the integrity of interactions between smooth muscle, nerves and IC, with compromised IC populations. Correlation between IC reduction and a hypercompliant phenotype suggests that disruption to bladder IC contribute to pathophysiological processes underpinning the dysfunctional SCI bladder.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures





References
-
- Kalejaiye O, Speakman MJ. Management of acute and chronic retention in men. Eur Urol. 2009;8:523–9.
-
- McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011a;202:233–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

