Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: a preliminary study
- PMID: 21883889
- PMCID: PMC3823081
- DOI: 10.1111/j.1582-4934.2011.01416.x
Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: a preliminary study
Abstract
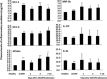
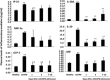
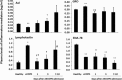
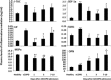
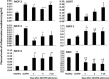
Systemic inflammation is a major factor influencing the outcome and quality of patient with chronic obstructive pulmonary disease (COPD) and acute exacerbations (AECOPD). Because of the inflammatory complexity, a great challenge is still confronted to optimize the identification and validation of disease-specific biomarkers. This study aimed at developing a new protocol of specific biomarker evaluation by integrating proteomic profiles of inflammatory mediators with clinical informatics in AECOPD patients, understand better their function and signal networks. Plasma samples were collected from healthy non-smokers or patients with stable COPD (sCOPD) or AECOPD on days 1 and 3 of the admission and discharging day (day 7-10). Forty chemokines were measured using a chemokine multiplex antibody array. Clinical informatics was achieved by a Digital Evaluation Score System (DESS) for assessing severity of patients. Chemokine data was compared among different groups and its correlation with DESS scores was performed by SPSS software. Of 40 chemokines, 30 showed significant difference between sCOPD patients and healthy controls, 16 between AECOPD patients and controls and 13 between AECOPD patients and both sCOPD and controls, including BTC, IL-9, IL-18Bpa, CCL22,CCL23, CCL25, CCL28, CTACK, LIGHT, MSPa, MCP-3, MCP-4 and OPN. Of them, some had significant correlation with DESS scores. There is a disease-specific profile of inflammatory mediators in COPD and AECOPD patients which may have a potential diagnostics together with clinical informatics of patients. Our preliminary study suggested that integration of proteomics with clinical informatics can be a new way to validate and optimize disease-special biomarkers.
© 2011 The Authors Journal compilation © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. - PubMed
-
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–20. - PubMed
-
- Donaldson GC, Seemungal TA, Patel IS, et al. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22:931–6. - PubMed
-
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

