Two separate Ni(2+) -sensitive voltage-gated Ca(2+) channels modulate transretinal signalling in the isolated murine retina
- PMID: 21883984
- PMCID: PMC3274955
- DOI: 10.1111/j.1755-3768.2011.02167.x
Two separate Ni(2+) -sensitive voltage-gated Ca(2+) channels modulate transretinal signalling in the isolated murine retina
Abstract
Purpose: Light-evoked responses from vertebrate retinas were recorded as an electroretinogram (ERG). The b-wave is the most prominent component of the ERG, and in the bovine retina its NiCl(2) -sensitive component was attributed to reciprocal signalling by pharmacoresistant R-type voltage-gated Ca(2+) channels, which similar to other voltage-dependent Ca(2+) channels trigger and control neurotransmitter release. The murine retina has the great advantage that the effect of gene inactivation for Ni(2+) -sensitive Ca(2+) channels can be analysed to prove or disprove that any of these Ca(2+) channels is involved in retinal signalling.
Methods: Superfused retinas from different murine genotypes lacking either one or both highly Ni(2+) -sensitive voltage-gated Ca(2+) channels were used to record their ex vivo ERGs.
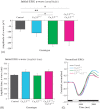
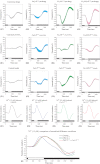
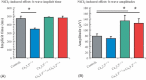
Results: The isolated retinas from mice lacking Ca(v)2.3 R-type or Ca(v)3.2 T-type or both voltage-gated Ca(2+) channels were superfused with a NiCl(2) (15 μm) containing nutrient solution. The change in the b-wave amplitude and implicit time, caused by NiCl(2), was calculated as a difference spectrum and compared to data from control animals. From the results, it can be deduced that Ca(v)2.3 contributes rather to a later component in the b-wave response, while in the absence of Ca(v)3.2 the gain of Ni(2+) -mediated increase in the b-wave amplitude is significantly increased, probably due to a loss of reciprocal inhibition to photoreceptors. Thus, each of the Ni(2+)-sensitive Ca(2+) channels contributes to specific features of the b-wave response.
Conclusion: Both high-affinity Ni(2+)-sensitive Ca(2+) channels contribute to transretinal signalling. Based on the results from the double knockout mice, additional targets for NiCl(2) must contribute to transretinal signalling, which will be most important for the structurally similar physiologically more important heavy metal cation Zn(2+).
© 2011 The Authors. Acta Ophthalmologica © 2011 Acta Ophthalmologica Scandinavica Foundation.
Figures


References
-
- Albanna W, Banat M, Albanna N, et al. Longer lasting electroretinographic recordings from the isolated and superfused murine retina. Graefes Arch Clin Exp Ophthalmol. 2009;247:1339–1352. - PubMed
-
- Ball SL, Gregg RG. Using mutant mice to study the role of voltage-gated calcium channels in the retina. Adv Exp Med Biol. 2002;514:439–450. - PubMed
-
- Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. - PubMed
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, et al. Loss-of-function mutations in a calcium-channel a1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998;19:264–267. - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

