Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity
- PMID: 21884167
- PMCID: PMC7165622
- DOI: 10.1111/j.1600-065X.2011.01048.x
Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity
Abstract
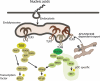
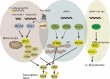
Host cells trigger signals for innate immune responses upon recognition of conserved structures in microbial pathogens. Nucleic acids, which are critical components for inheriting genetic information in all species including pathogens, are key structures sensed by the innate immune system. The corresponding receptors for foreign nucleic acids include members of Toll-like receptors, RIG-I-like receptors, and intracellular DNA sensors. While nucleic acid recognition by these receptors is required for host defense against the pathogen, there is a potential risk to the host of self-nucleic acids recognition, thus precipitating autoimmune and autoinflammatory diseases. In this review, we discuss the roles of nucleic acid-sensing receptors in guarding against pathogen invasion, discriminating between self and non-self, and contributing to autoimmunity and autoinflammatory diseases.
© 2011 John Wiley & Sons A/S.
Figures

References
-
- Kawai T, Akira S. Toll‐like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011;34:637–650. - PubMed
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–820. - PubMed
-
- Roberts RM, Liu L, Guo Q, Leaman D, Bixby J. The evolution of the type I interferons. J Interferon Cytokine Res 1998;18:805–816. - PubMed
-
- Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science 2011;332:1093–1097. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

