dsRNA sensors and plasmacytoid dendritic cells in host defense and autoimmunity
- PMID: 21884168
- PMCID: PMC3170087
- DOI: 10.1111/j.1600-065X.2011.01049.x
dsRNA sensors and plasmacytoid dendritic cells in host defense and autoimmunity
Abstract
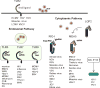
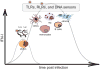
The innate immune system detects viruses through molecular sensors that trigger the production of type I interferons (IFN-I) and inflammatory cytokines. As viruses vary tremendously in size, structure, genomic composition, and tissue tropism, multiple sensors are required to detect their presence in various cell types and tissues. In this review, we summarize current knowledge of the diversity, specificity, and signaling pathways downstream of viral sensors and ask whether two distinct sensors that recognize the same viral component are complementary, compensatory, or simply redundant. We also discuss why viral sensors are differentially distributed in distinct cell types and whether a particular cell type dominates the IFN-I response during viral infection. Finally, we review evidence suggesting that inappropriate signaling through viral sensors may induce autoimmunity. The picture emerging from these studies is that disparate viral sensors in different cell types form a dynamic and integrated molecular network that can be exploited for improving vaccination and therapeutic strategies for infectious and autoimmune diseases.
© 2011 John Wiley & Sons A/S.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

